Remy
Administrator
I learned a new word today...immunosenescence. It basically means the gradual decline of the immune system due to aging and I think it has a lot of relevance to MECFS.
"Several studies have highlighted that immunosenescence occurs as the result of a chronic hyperstimulation of both adaptive and innate immune system components[5, 6, 7, 8], which together with the accumulation of molecular scars due to the progressive deterioration of molecular components and pathways[9] leads to a peculiar immune status characterized by a global loss of efficiency (Figure 1)."
So the immune system is underactive and overactive all at the same time...well, there's a concept!
[bimg=no-lightbox]https://static-content.springer.com/image/art%3A10.1186%2F1742-4933-10-9/MediaObjects/12979_2012_Article_132_Fig1_HTML.jpg[/bimg]
Mady Hornig seems to have (had?) immunosenescence in her line of vision too and it makes sense when you look at the changes that occur with regards to inflammatory cytokines and the adaptive and innate immune system. Even the hormonal changes and mast cell issues are accounted for in an MECFS paradigm where a rapid state of immunosenescence goes all wrong.
Cells that should die (apoptosis) and get cleared away by the immune system back up and become problematic. Inflammatory cytokines get secreted. Macrophages don't work well. NK cells decline. B cells decline and T cells do not differentiate properly.
This all sounds REALLY familiar to me!
Even more interestingly, the compounds that seem to clear up the problem are the tyrosine kinase inhibitors. The very same weirdo cancer drugs that Dr Afrin has found to be helpful in treating mast cell disease. What if Dr Afrin is really treating a symptom of an underlying disorder of immunosenescence?
BTW, quercetin looks to be really helpful as well. I'm about thisclose to saying that everyone with MECFS needs to be taking quercetin for all the good things it can do.
ProHealth gives us a nice simple explanation of this complicated process:
I wonder if anyone has heard of this Dr Marian Laderoute? She seems to run some sort of private lab in Canada...but she has also written recently on this subject and it's fascinating (if very long). I've trimmed an excerpt below.
"Several studies have highlighted that immunosenescence occurs as the result of a chronic hyperstimulation of both adaptive and innate immune system components[5, 6, 7, 8], which together with the accumulation of molecular scars due to the progressive deterioration of molecular components and pathways[9] leads to a peculiar immune status characterized by a global loss of efficiency (Figure 1)."
So the immune system is underactive and overactive all at the same time...well, there's a concept!
[bimg=no-lightbox]https://static-content.springer.com/image/art%3A10.1186%2F1742-4933-10-9/MediaObjects/12979_2012_Article_132_Fig1_HTML.jpg[/bimg]
Mady Hornig seems to have (had?) immunosenescence in her line of vision too and it makes sense when you look at the changes that occur with regards to inflammatory cytokines and the adaptive and innate immune system. Even the hormonal changes and mast cell issues are accounted for in an MECFS paradigm where a rapid state of immunosenescence goes all wrong.
Cells that should die (apoptosis) and get cleared away by the immune system back up and become problematic. Inflammatory cytokines get secreted. Macrophages don't work well. NK cells decline. B cells decline and T cells do not differentiate properly.
This all sounds REALLY familiar to me!
Even more interestingly, the compounds that seem to clear up the problem are the tyrosine kinase inhibitors. The very same weirdo cancer drugs that Dr Afrin has found to be helpful in treating mast cell disease. What if Dr Afrin is really treating a symptom of an underlying disorder of immunosenescence?
BTW, quercetin looks to be really helpful as well. I'm about thisclose to saying that everyone with MECFS needs to be taking quercetin for all the good things it can do.
ProHealth gives us a nice simple explanation of this complicated process:
A healthy immune system can help remove senescent cells as part of its normal cellular housekeeping. This process is important in cancer prevention.7,16 As bodies age, the immune system itself begins to age via a process known as immune senescence. As a result, removal of senescent cells begins to fail. This leads to an acceleration of aging, and ultimately, an increase in the risk of most age-associated conditions, including cancer.16
By selectively eliminating senescent cells so that they do not “clog up” our body’s processes, it is possible to support or even regain youthful function in every organ and tissue. This was shown in a compelling fashion in a 2011 study from the Mayo Clinic, using a mouse model of rapidly accelerated aging similar to the disease progeria in humans.17
Progeria is a rare disease of accelerated aging. It is the result of a gene mutation that produces premature accumulation of senescent cells, and causes young children to age so rapidly that most die before age 15, commonly of atherosclerosis or other age-related processes.18,19
The Mayo researchers transplanted into the rapidly aging mouse model a specialized gene that selectively eliminated senescent cells.17 They found that, by removing senescent cells in tissues such as fat, skeletal muscles, and the eye, they could significantly delay onset of age-related disorders in those tissues. These included muscle loss, cataracts, and depletion of subcutaneous (under-the-skin) fat. Perhaps still more exciting, clearance of senescent cells even in late life slowed the progression of age-related disorders already in progress.
I wonder if anyone has heard of this Dr Marian Laderoute? She seems to run some sort of private lab in Canada...but she has also written recently on this subject and it's fascinating (if very long). I've trimmed an excerpt below.
Published on December 29, 2015
Author: Marian P Laderoute
Specialty: Clinical Immunology, Immunology, Infectious Diseases, Oncology, Biotechnology
Institution: Immune System Management Clinic and Lab
Address: Ottawa, Ontario, K1S 5R5, Canada
Abstract: The majority of chronic diseases in the aging adult are thought to relate to immune aging characterized by dominant immunosuppression and paradoxically, concomitant inflammation.
This is known collectively as immunosenescence.
The main change thought to be controlling immune aging is the age-related decline in dehydroepiandrosterone (DHEA) and corresponding increase in cortisol; the net effect which decreases the DHEA/cortisol ratio.
Exactly how this translates to immunosuppression and concomitant inflammation remains unclear.
Recently a new component of the human innate immune system has been discovered. Human endogenous retrovirus K102 (HERV-K102) is a replication-competent foamy retrovirus unique to humans which has been implicated in chronic diseases.
Accumulating evidence suggests that HERV-K102 may defend the host against viral infections, as well as against breast and other cancers.
Particles are produced in activated monocytes and released into vacuoles but do not bud through the cell surface. This renders macrophages foamy, while the release of particles is only through cell lysis.
New evidence presented here suggests DHEA but not DHEA-S may specifically bind and inactivate alpha-fetoprotein (AFP). AFP is a well-established immunosuppressive factor which importantly, also blocks cell lysis induction in macrophages through the 67 kilodalton (kD) AFP receptor (AFPr).
Here, it is proposed that a decreased DHEA/cortisol ratio may favor the accumulation of foamy macrophages reflecting the cortisol induction of HERV-K102 particle production concomitant with the blocked release of particles by secreted AFP.
This is a new paradigm to explain how cortisol-mediated immunosenescence can result in the persistence of foamy macrophages, and how this relates to risk of chronic disease.
Introduction
Immunosenescence is a term used to describe the overall diminished functioning or dysregulation of the immune system associated with aging (Baylis et al., 2013), the details of which have been described elsewhere (Aw et al., 2007; Lindstrom and Robinson, 2010; Hsu et al., 2005). Paradoxically, immunosenescence also incorporates a propensity for upregulation of the inflammatory response, which has been called ‘inflammaging’ as coined by Franceschi et al. in 2000. While the precise etiology of inflammaging remains largely unknown (Franceschi and Campisi, 2014), it is characterized by increased levels of IL-1, IL-6, TNF-alpha and CRP and reduced IL-10 in the blood (Baylis et al., 2013; Bueno et al., 2014; Lindstrom and Robinson, 2010) implying activated macrophages/dendritic cells in the process. Diseases linked with inflammaging include cardiovascular disease, cancer, dementia, Alzheimer’s disease, autoimmune diseases, type-2 diabetes, HIV-1, and increased vulnerability to infectious disease (Baylis et al., 2013; Heffner, 2011; Fulop et al., 2013; Kuller et al., 2008; Guaraldi et al., 2011; Lo and Plutzky, 2012; Dillon et al., 2013; Gruver et al., 2007).
Immunosenescence is most closely related to adrenal aging (Valenti, 2004; Heffner, 2011; Lois et al., 2014; Giefing-Kroll et al., 2015). As humans age, the levels of dehydroepiandrosterone (DHEA) decrease and cortisol increase (Buford and Willoughby, 2008; Baylis et al., 2013), and together they decrease the DHEA/cortisol ratio. DHEA is also known to blunt the cortisol stress response and to reduce cortisol levels (Miyake et al., 2014). Accordingly, as we age we do not handle stress as well as before when younger. In addition to increased serum cortisol, macrophages express increased glucocorticoid receptor mRNA associated with aging or stress (Kizaki et al., 2002). Decreased DHEA/cortisol ratios in addition to reduced DHEA are detected in a number of chronic diseases (Baylis et al., 2013) including those which generally affect younger adults, such as HIV-1 (Christeff et al., 2000; Dillon et al., 2013). Premature immunosenescence is known to occur with chronic psychological stress, chronic inflammation or exposure to certain persistent viral infections, with the latter appearing to elevate cortisol (Bauer et al., 2015).
The relative balance of Th1 versus Th2 responses also shifts with aging towards the latter and this imbalance is also seen in psychological stress (Heffner, 2011). A Th2 response is associated with autoimmune IgG antibody production, which increases with age (Nagele et al., 2013). Immunosenescence leads to dysregulation of the immune system and thus, autoimmune disease with autoimmune antibodies frequently increases with aging consistent with its Th2 dominance (Mills, 2015). Sharon and Mason (2015) assert that autoimmune disease generally is associated with immunodeficiency and the inability to clear pathogens and highlights the notion of dysregulated immunity in immunosenescence.
There are three main types of cells which mediate non-specific immunosuppression (Baert et al., 2015; Steinberg et al., 2014): the non-antigen specific suppressor T cells now referred to as regulatory T cells (Tregs), myeloid derived suppressor cells (MDSC) which are precursors to macrophages/dendritic cells (Draghiciu et al., 2015), and immunosuppressive tumor associated macrophages (TAMs). Interestingly, these immunosuppressive cell types can also display inflammatory properties (Bowdish, 2013; Schmitt et al., 2012; Baert et al., 2015) and as alluded to, may be associated with autoimmunity (Crook and Liu, 2014; Stevenson et al., 2014; Schmitt et al., 2012).
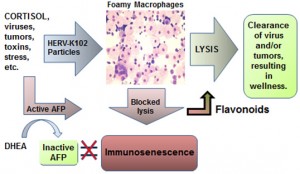
Figure 1. Model for how cortisol may activate HERV-K102 particle production producing foamy macrophages and how blocked release may lead to immunosenescence and chronic diseases.
In this review article a new paradigm is being proposed in an attempt to explain how both immunosuppression and inflammatory activation in macrophages may be achieved by excess, unopposed cortisol associated with aging. Cortisol is proposed to directly induce particle production of human endogenous retrovirus K102 (HERV-K102) in monocytes/macrophages rendering them foamy and at the same time to transactivate expression of alpha-fetoprotein (AFP) mRNA. Secreted AFP then is postulated to bind back to the 67 kD AFP receptor (AFPr) expressed on partially activated macrophages. This blocks cell death induction thereby directly preventing the release of HERV-K102 particles (see Figure 1) and may also prevent the further differentiation of macrophages. The blocked release of HERV-K102 particles in foamy macrophages by AFP is hypothesized to result both in diminished protection by HERV-K102 against infectious agents and tumors, and the lingering of foamy macrophages. It is the persistence of these foamy macrophages which contributes to inflammation and it is the release of AFP which contributes to immunosuppression. Together these are postulated to cause “immunosenescence” where the immune system is dysfunctional. In this state, the host is at higher risk of inflammatory diseases such as autoimmune conditions, cancer, neurological disorders, and most directly an increased risk of atherosclerosis. As will also be developed below, in part the reduction in dehydroepiandrosterone (DHEA) which is also associated with stress and/or aging and which is commonly found in chronic diseases, provides less ability of the host to control or inhibit the active levels of AFP in the system further contributing to immunosenescence.
Summary
It has been suggested that age-related immune dysfunction may at least in part explain the aging process (Fulop et al., 2013; 2014). However, no single theory explains all aspects of aging. Indeed, the causes of immunosenescence which involves immunosuppression and inflammation, remain to be deciphered (Jenny, 2012).
This treatise was not an attempt to address aging per se, but was to try to explain how the age-related changes in the DHEA/cortisol level might be associated with the age-associated risk of chronic disease involving immunosenescence. The salient ideas behind this paradigm are that the induction of HERV-K102 particles and AFP expression is mediated by cortisol. Due to stress on the system and/or immune reactivity; tumors, infectious agents, toxins or trauma, may also indirectly increase cortisol levels. In order to explain the link with aging, the blocked release of HERV-K102 particles from foamy macrophages may be favored when DHEA levels may be insufficient to inactivate AFP. DHEA levels are known to diminish with aging and are depressed in patients with chronic disease. In aged or non-healthy adults, insufficient DHEA allows for the persistence of foamy macrophages as there is less DHEA in the system to inactivate AFP. These persistent foamy macrophages presumably release inflammatory mediators as well as immunosuppressive molecules like active AFP, although this remains to be directly shown. This then is postulated to directly contribute to immunosenescence and the dysfunction of the immune system.
It is known that dietary antioxidants such as flavonoids and vitamin C which may also behave as nutraceuticals, may delay or ameliorate symptoms of aging (Peng et al., 2014). Accordingly, plausible paradigms to explain immunosenescence would need to have the role of flavonoids addressed which was achieved here. It is suggested flavonoids may lower AFP activity and/or expression and there is some evidence in the literature to support this (Saleem et al., 2013). It is also notable that various flavonoids may serve to down-modulate cortisol activity by preventing the conversion of inactive cortisone to cortisol (Hintzpeter et al., 2014; Tagawa et al., 2015).
Conclusions
The paradigm presented here of cortisol-mediated HERV-K102 blocked particle release in foamy macrophages as well as the association of a role of AFP in immunosenescence may be invaluable to understanding the risk of chronic illnesses with age. If correct, it is expected to drive rational prevention and treatment strategies, for example as suggested by Fantidis (2010). On the other hand, the current approach of using immunosuppressive therapies to treat age-associated autoimmune diseases and cancer, or to prevent atherosclerosis and heart disease such as by statins, may need to be revisited. It will therefore be of high priority to determine whether or not HERV-K102 particles are oncolytic or virolytic at least under permissive conditions. It will be equally important to address whether these particles in plasma are associated with remission of chronic diseases as has been shown in a limited number of patients addressed so far. It would also be useful to develop better clinical methods for unveiling the presence of active, immunosuppressive AFP in serum in patients with chronic or active illnesses to help determine whether AFP is implicated or not, in a wide variety of age-associated chronic diseases.












