In a comment Jim noted that the course of Rituximab in the Norwegian trials would cost about $120,000!  eek
eek ...Even with insurance it would cost at least $5K....
...Even with insurance it would cost at least $5K.... banghead
banghead .
.
So I went on the hunt for generics. It turns out that the type of drug Rituximab is - a biologic - is different. There is hope for a generic but some barriers standing in the way indicate it will take some time. Still, this is a commonly used drug with lots of interest in it. I imagine drug companies would be frothing at the mouth at the chance to make it. This is what a Leukemia site said:
That wasn't too exciting but here's some good news.
Rituxian's patents in Europe expired in 2013. It generated $7.6 billion in sales in 2013 - twenty percent of Roche's sales, and big pharma companies including Pfizer (NYSE:PFE), Novartis (NYSE:NVS), Merck (NYSE:MRK), Teva (NYSE:TEVA) and Boehringer Ingelheim are just a few of the companies that initiated programs to launch a biosimilar Rituxan. (!). Rituximab generics are expected to enter the European market in 2015.
It's definitely coming..
(click to enlarge)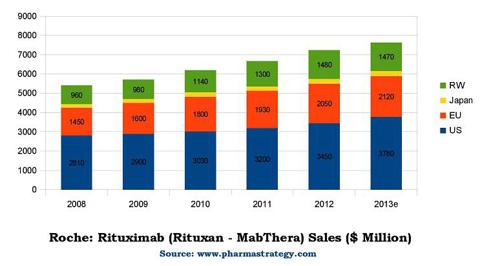
Novartis's drug is completing phase 3 trials in a lymphoma and phase 2 clinical in patients with rheumatoid arthritis. Boehringer Ingelheim has reached phase 3 clinical in patients with rheumatoid arthritis Pfizer was in phase 2 trial in rheumatoid arthritis.
It's a difficult drug to manufacture.
Financial Impact
In the US Rituxan's patent will expire in 2018. Rituximab's manufacture Roche is already working on an upgrade Obinutuzumab to replace Rituximab.
So I went on the hunt for generics. It turns out that the type of drug Rituximab is - a biologic - is different. There is hope for a generic but some barriers standing in the way indicate it will take some time. Still, this is a commonly used drug with lots of interest in it. I imagine drug companies would be frothing at the mouth at the chance to make it. This is what a Leukemia site said:
Because Rituxan (rituximab) is considered a “biologic” medication, it is subject to rules and laws that prevent any generic versions from being manufactured.
However, these laws will probably be changed to allow for generic biologics to be made. When these rules change, patents could still protect Rituxan from generic competition, so it’s hard to say when a generic form of the drug could become available.
When Will Generic Rituxan Be Available?
Although new laws have cleared a path for the possibility of generic biologics, there is still quite a bit of rulemaking to be done. However, even once all the official business of allowing generic biologics is completed, it is likely that Rituxan will still be protected from generic competition by patents. It is not exactly clear when the patents expire, as the United States Food and Drug Administration (FDA) does not yet list applicable patents for biologics, as it does for other medications.
That wasn't too exciting but here's some good news.
Rituxian's patents in Europe expired in 2013. It generated $7.6 billion in sales in 2013 - twenty percent of Roche's sales, and big pharma companies including Pfizer (NYSE:PFE), Novartis (NYSE:NVS), Merck (NYSE:MRK), Teva (NYSE:TEVA) and Boehringer Ingelheim are just a few of the companies that initiated programs to launch a biosimilar Rituxan. (!). Rituximab generics are expected to enter the European market in 2015.
It's definitely coming..
(click to enlarge)

Novartis's drug is completing phase 3 trials in a lymphoma and phase 2 clinical in patients with rheumatoid arthritis. Boehringer Ingelheim has reached phase 3 clinical in patients with rheumatoid arthritis Pfizer was in phase 2 trial in rheumatoid arthritis.
It's a difficult drug to manufacture.
Financial Impact
CompanyTickerP/EP/SMkt Cap
Merck MRK 19.7 2.9 $125 Billion
Novartis NVS 19.7 3.5 $184 Billion
Pfizer PFE 20.7 3.4 $202 Billion
Roche RHHBY.OB 18.6 4.6 $232 Billion
Teva TEVA 83 1.9 $34 Billion
Merck MRK 19.7 2.9 $125 Billion
Novartis NVS 19.7 3.5 $184 Billion
Pfizer PFE 20.7 3.4 $202 Billion
Roche RHHBY.OB 18.6 4.6 $232 Billion
Teva TEVA 83 1.9 $34 Billion
In the US Rituxan's patent will expire in 2018. Rituximab's manufacture Roche is already working on an upgrade Obinutuzumab to replace Rituximab.












