Seanko
Well-Known Member
Dr Elizabeth Unger & colleagues at the CDC in Atlanta have published a paper on Telomere shortening in ME/CFS in the FASEB biology journal
Telomere Length Analysis in Chronic Fatigue Syndrome
Below is a readable summary from ME Research UK
Telomere Shortening
Telomeres are ‘caps’ of DNA and protein located at the end of chromosomes to protect them from deteriorating or becoming fused with other chromosomes when cells are dividing. Structurally, they consist of a region of hundreds or even thousands of repetitive sequences of nucleotide letters, usually repeats of the sequence TTAGGG.
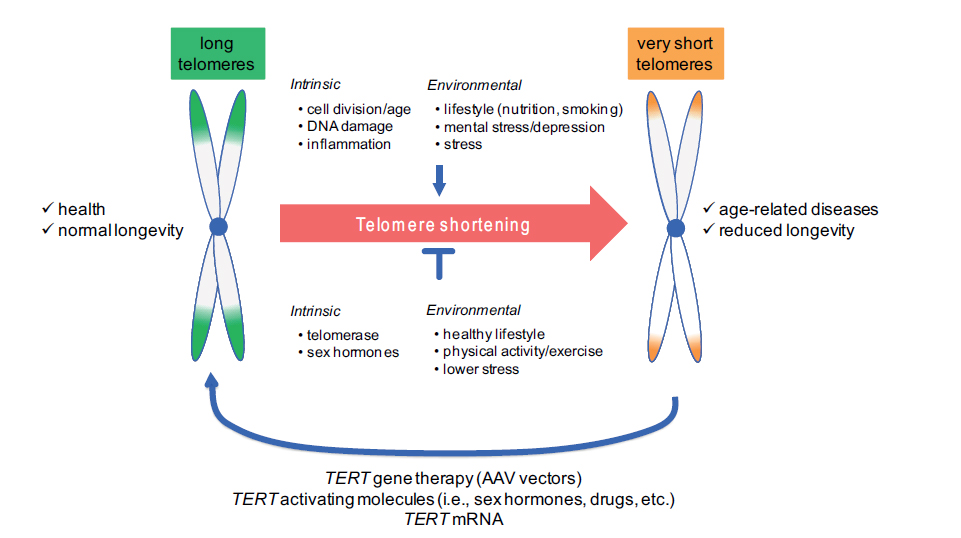
One of the characteristics of telomeres is that they become shorter during the ageing process; they shorten every time a cell divides – human blood cells shorten by 30−70 base pairs per year on average. This weakens the structural integrity of cells and causes them to age and die faster. It remains unknown whether telomere shortening is simply a sign of ‘cellular age’ or whether it contributes to the ageing process more directly. We do know, however, that telomeres are shorter than usual in patients with diseases like cancer, osteoporosis and the cardiovascular disorders, even when the effect of ageing is taken into account.
Recently, evidence has come to light that telomeres are also shorter than normal in people with ME/CFS. The study, presented as an abstract at the Experimental Biology 2016 Meeting and published this month in The FASEB Journal (read more), comes from Prof Unger’s group at the CDC in Atlanta. The researchers isolated DNA from whole blood samples from 639 participants who had taken part in the 2004 Georgia CFS Surveillance study (read more), one of the two population-based longitudinal studies of CFS undertaken by the CDC in the past 20 years. From each sample, relative telomere length was measured using real-time PCR. Using questionnaire-based methods, 64 participants were classified as fulfilling all the criteria for Fukuda 1994 CFS; 77 fulfilled all the criteria but had other exclusionary conditions; 302 fulfilled only some of the criteria (‘fatigue’ group in this case), and 196 met none of the criteria (‘healthy controls’ in this case).
Overall, telomere lengths were found to be significantly longer in the healthy controls than in the Fukuda 1994 CFS patients or in the group of ‘fatigue’ patients, and these differences remained significant after statistical adjustment for differences between groups. Based on adjusted group means, telomere length was shorter by 593 and 508 base pairs in the CFS and ‘fatigue’ groups, respectively, compared with the controls. As expected, there was a correlation between telomere length and age in the total sample. Given this evidence, the authors suggest that CFS should be considered a condition of ‘telomere shortening’.
As shortened telomeres are also found in a range of other chronic diseases, including cancer (read more), diabetes, Alzheimer’s disease and Parkinson’s disease, the phenomenon is most likely associated with chronic illness per se, rather than with ME/CFS in particular. It is intriguing to note, however, that telomeres are highly susceptible to oxidative stress, which is known to damage DNA; the higher the cellular oxidative stress levels the greater the degree of shortening (read more). Given that ME Research UK-funded work has found high levels of oxidative stress and associated arterial stiffness to be a feature in ME/CFS patients (read more), it may be that telomere shortening is intimately linked with ongoing inflammatory processes.
Though telomere length decreases with age, it seems that the process is not inevitable and that they can also increase in length. At present, scientists are exploring interventions to lengthen telomeres. Telomerase is the enzyme that repairs shortened or dysfunctional telomeres, and various telomerase-activating drugs are under development, with some success as recent work on blood disorders has shown (read more). Also, statins seem to have a protective role against telomere shortening, and lifestyle factors may have an important role to play. For example, telomere length is positively linked with nutritional status in human and animal studies (see a review), possibly through the effects of various nutrients on reducing inflammation and oxidative stress, and a study in 2013found that lifestyle changes (a plant-based diet, moderate exercise, stress reduction and weekly group support) increased telomere length by about 10% in men with prostate cancer.
Of course, it is too early to know the precise meaning of telomere shortening in ME/CFS patients and what, if anything, should be done to combat it, but these intriguing findings add to the evidence of an underlying disease process in people with the illness, and we await the publication of the full scientific paper with great interest.
Telomere Length Analysis in Chronic Fatigue Syndrome
Below is a readable summary from ME Research UK
Telomere Shortening

Telomeres are ‘caps’ of DNA and protein located at the end of chromosomes to protect them from deteriorating or becoming fused with other chromosomes when cells are dividing. Structurally, they consist of a region of hundreds or even thousands of repetitive sequences of nucleotide letters, usually repeats of the sequence TTAGGG.
One of the characteristics of telomeres is that they become shorter during the ageing process; they shorten every time a cell divides – human blood cells shorten by 30−70 base pairs per year on average. This weakens the structural integrity of cells and causes them to age and die faster. It remains unknown whether telomere shortening is simply a sign of ‘cellular age’ or whether it contributes to the ageing process more directly. We do know, however, that telomeres are shorter than usual in patients with diseases like cancer, osteoporosis and the cardiovascular disorders, even when the effect of ageing is taken into account.
Recently, evidence has come to light that telomeres are also shorter than normal in people with ME/CFS. The study, presented as an abstract at the Experimental Biology 2016 Meeting and published this month in The FASEB Journal (read more), comes from Prof Unger’s group at the CDC in Atlanta. The researchers isolated DNA from whole blood samples from 639 participants who had taken part in the 2004 Georgia CFS Surveillance study (read more), one of the two population-based longitudinal studies of CFS undertaken by the CDC in the past 20 years. From each sample, relative telomere length was measured using real-time PCR. Using questionnaire-based methods, 64 participants were classified as fulfilling all the criteria for Fukuda 1994 CFS; 77 fulfilled all the criteria but had other exclusionary conditions; 302 fulfilled only some of the criteria (‘fatigue’ group in this case), and 196 met none of the criteria (‘healthy controls’ in this case).
Overall, telomere lengths were found to be significantly longer in the healthy controls than in the Fukuda 1994 CFS patients or in the group of ‘fatigue’ patients, and these differences remained significant after statistical adjustment for differences between groups. Based on adjusted group means, telomere length was shorter by 593 and 508 base pairs in the CFS and ‘fatigue’ groups, respectively, compared with the controls. As expected, there was a correlation between telomere length and age in the total sample. Given this evidence, the authors suggest that CFS should be considered a condition of ‘telomere shortening’.
As shortened telomeres are also found in a range of other chronic diseases, including cancer (read more), diabetes, Alzheimer’s disease and Parkinson’s disease, the phenomenon is most likely associated with chronic illness per se, rather than with ME/CFS in particular. It is intriguing to note, however, that telomeres are highly susceptible to oxidative stress, which is known to damage DNA; the higher the cellular oxidative stress levels the greater the degree of shortening (read more). Given that ME Research UK-funded work has found high levels of oxidative stress and associated arterial stiffness to be a feature in ME/CFS patients (read more), it may be that telomere shortening is intimately linked with ongoing inflammatory processes.
Though telomere length decreases with age, it seems that the process is not inevitable and that they can also increase in length. At present, scientists are exploring interventions to lengthen telomeres. Telomerase is the enzyme that repairs shortened or dysfunctional telomeres, and various telomerase-activating drugs are under development, with some success as recent work on blood disorders has shown (read more). Also, statins seem to have a protective role against telomere shortening, and lifestyle factors may have an important role to play. For example, telomere length is positively linked with nutritional status in human and animal studies (see a review), possibly through the effects of various nutrients on reducing inflammation and oxidative stress, and a study in 2013found that lifestyle changes (a plant-based diet, moderate exercise, stress reduction and weekly group support) increased telomere length by about 10% in men with prostate cancer.
Of course, it is too early to know the precise meaning of telomere shortening in ME/CFS patients and what, if anything, should be done to combat it, but these intriguing findings add to the evidence of an underlying disease process in people with the illness, and we await the publication of the full scientific paper with great interest.













