Cerebrospinal fluid studies are rare in chronic fatigue syndrome (ME/CFS), and even rarer are those that make a clear difference. The “Cerebrospinal fluid immune phenotyping reveals distinct immunotypes of myalgic encephalomyelitis/chronic fatigue syndrome“, from Akiko Iwasaki’s Yale lab, and led by Victoria Bastos and Kerrie Greene, definitely brought something new to the field.
The study focused on cerebrospinal fluid and plasma (blood)-assessed cytokines, hormones, matrix metalloproteinases, and autoantibodies, and sought evidence of pathogens. It included 39 healthy controls and 40 people with ME/CFS (Canadian Consensus Criteria).
Results
The finding that people with ME/CFS haven’t been more exposed to pathogens was not unexpected given past study findings. No differences were found in IgG autoantibodies in either the cerebrospinal fluid or the plasma. Only subtle differences were found in antibodies overall.
The most interesting result emerged from a machine learning technique that identified two clusters based on MMP and cytokine levels. Despite the fact that both clusters of patients had identical symptoms and functioning, a rather discerning look under the hood focused on the “matrix metalloproteinases” in the cerebral spinal fluid found evidence that two clusters were present.
The Spinal/Craniocervical Instability Cluster (?)

Two clusters – one apparently normal and one with significant cerebral spinal fluid abnormalities.
These researchers chose their tests well. The first cluster, which contained about 1/4 of the patients, had a significantly higher “signature” of no less than three of the four matrix metalloproteinases (MMP-1, MMP-2, and MMP-10) assessed.
The MMPs could be called the great “degraders”. These enzymes digest the proteins (collagen, elastin, gelatin, etc.) in the extracellular matrix (ECM; read, the connective tissues).
The extracellular matrix (ECM) basically keeps the tissues, organs, and blood vessels where they should be. Saggy blood vessels, irritated nerves, and spinal problems could all be examples of damaged connective tissue that no longer keeps the tissues in the right place in diseases like ME/CFS.
By breaking these proteins down into smaller fragments, MMPs assist with wound healing, blood vessel formation, and the entry of immune cells into the matrix to chase down pathogens and clean up injuries.
Balance is everything in the body, though, and overactive MMPs have been implicated in a wide array of diseases including atherosclerosis, peripheral vascular disease, multiple sclerosis, and fibrotic diseases.
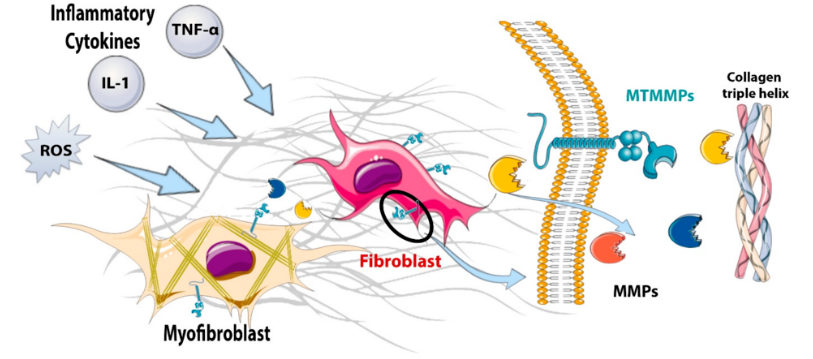
A model of MMP degradation. It starts at the left with inflammation, and ends with the MMPs on the right gobbling up the collagen. (from Venugopal, H. et al. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 2022, 11, 1386.)
Each of the abnormally elevated MMPs degrades different things: MMP-1 breaks down the main structural protein in the connective tissues; MMP-2 targets basement membrane components making the way for new blood vessels and immune cells; MMP-10 is mainly produced during inflammation. Both MMP-1 and two are known to target the central nervous system. (They did not assess a metalloproteinase called MMP-9, which is often associated with central nervous system issues, including neuroinflammation.)
The high cerebrospinal fluid MMP levels in the first cluster suggested that the tissues in their central nervous system were getting hit hard. Inflammation, a weakened blood-brain barrier, problems with nerve function, and, interestingly, conditions such as craniocervical instability could all be associated with excessive MMP activity.
Usually we think of brain imaging with regard to CCI. This is the first time a lab study has suggested they found indirect evidence of it. Interestingly, they appear to believe the CCI probably triggered the increased MMP and cytokine levels.
“We postulate that instability and obstructions at the craniocervical junction may contribute to impaired movement and production of CSF, in turn altering MMP or cytokine levels in either cluster.”
I asked AI Perplexity whether “high levels of matrix metalloproteinases could (also) contribute to craniocervical instability” and it believed they could. (Note in the answer how quickly the monocytes – an innate immune cell we’ve heard so much about recently – show up in their active form (macrophages). AI Perplexity reported that:
“Overactive MMPs, secreted in response to inflammation or by inflammatory macrophages, can degrade these connective tissues by attacking collagen and other structural proteins. This degradation can lead to ligament laxity and joint instability, which are hallmarks of CCI (as well as intervertebral disc degeneration, facet joint laxity, and other degenerative spinal conditions.”
Inflammation Too?
With evidence suggesting that the extracellular matrix in this cluster was being degraded, the researchers checked to see if inflammation was also present, and it was – in spades. Higher levels of no less than 8 pro-inflammatory cytokines (IL-8, IL-15, FLT-3L, MCP-1, M-CSF, SCF, IL-10, and IL-5) were found in Cluster 1.
The elevated levels of IL-15 caught the researchers’ eyes. IL-15 regulates the CX3CR1 receptor, CX3CRI, which determines whether fractalkine plays nice and tamps down inflammation or turns nasty and ramps it up, weakens the blood-brain barrier, and tweaks the nerves. Increased levels of an MMP, which also cleaves fractalkine, also suggested that something was up with fractalkine.
Strange Associations
Fractalkine – The fact that fractalkine levels in the patients in cluster 1 were not correlated with the levels of the MMP cleaver (but were in cluster 2) suggested something was off in Cluster I. If I got it right, it appears that the fractalkine may be getting was getting stuck on the cellular membranes instead of being released into the fluid. Since fractalkine is a chemoattractant, it could be calling monocytes, neutrophils and T-cells to adhere to and attack the tissues in the central nervous system. Hence the possibility that “local damage” was present.
Eotaxin has shown up in past ME/CFS and fibromyalgia studies. (Image from Crump,-M.P.,-Rajarathnam,-K.,-Sykes,-B_Visualization- Astrojan_Wikimedia_Commons)
Eoxtaxin – Another strange lack of correlation with the MMPs showed up with eotaxin. By upregulating MMP-2 and/or by attracting eosinophils that release enzymes that break down proteins, eotaxin could be contributing to connective tissue damage. Eotaxin has been associated with neurodegeneration and impaired memory as well as with increased collagen deposition (fibrosis) in some diseases.
Two ME/CFS studies have found elevated eotaxin levels, including one that found “very strongly elevated” cerebrospinal fluid (CSF) levels.
Eotaxin has been more fully investigated in fibromyalgia (FM). At least three fibromyalgia studies also found elevated plasma or serum eotaxin, one of which proposed that eotaxin was triggering mast cell activation. Younger’s 2023 study, indicating that FM patients respond to an immune trigger with an exaggerated release of eotaxin, echoed the findings of a small 2016 study, which concluded that monocytes in FM released abnormally high levels of eotaxin. Elevated cerebrospinal eotaxin levels have also been found in a small study of neuropathic pain.
Additionally, a recent study assessing the neurobiological effects of a mild coronavirus and influenza infection in mice primarily focused on eotaxin (CCL11). Despite the fact that no virus was found in the brain, the study found prominently elevated cytokine profiles in the cerebrospinal fluid. Eotaxin stood out because, in contrast to the other immune factors, eotaxin levels continued to increase over time: levels were higher at 7 weeks than at 2 weeks.
The authors hypothesized a link between increased microglial reactivity in the hippocampus and damage to the myelin sheaths of nerves, and questioned whether other “subanatomical” regions might also be affected. They noted that “even small changes in myelination can exert profound effects on neural circuit dynamics and consequently on cognitive function”.
The authors concluded that even a mild COVID-19 infection can result in “profound and prolonged changed in cytokines within the central nervous system”. They noted that preclinical (non-human) studies suggest that anti-inflammatory and neuro-regenerative strategies “can restore neural plasticity” and “restore healthy cognition”.
One quite comprehensive study concluded, to the authors’ surprise, that the secretion of phosphatidylcholine lipids with very-long chain fatty acid acyl chains (VLCPCs) and long-chain saturated free fatty acids (FFAs) was driving astrocyte upregulation and neurotoxicity (neuroinflammation).
Treatment
TIMP1 interacting with an MMP. (Image by Valerie Ann Perez, 2016, Wikimedia Commons)
The authors suggested that Jak-Stat inhibitors that can cross the blood-brain barrier could help this group. A large Baricitinib long COVID trial is underway, and several other Jak-Stat inhibitors (ruxolitinib, filgotinib) have been proposed.
They did not mention matrix metalloproteinase inhibitors, but several may be able to knock down some of the MMPs (as well as MMP-9) found in this study. They include TIMP-1 and 2, GM6001 (ilomastat), batimastat (BB-94), prinomastat (AG3340 – MMP9 inhibitor), tanomastat (BAY 12-9566 – broad spectrum), rebimastat, decaliximab (GS5745), minocycline, and doxycycline.
Cluster 2
After all the to-do about Cluster 1, there wasn’t much to say about Cluster 2, except that it did not display the abnormalities the first cluster did. Oddly enough, Cluster 2 displayed the most joint hypermobilty (half the patients had it) – and also had a higher positivity to the SARS-CoV-2 coronavirus and the parvovirus.
The clues to the larger cluster do not, at least for now, appear to reside in the cerebrospinal fluid.
Patient-Funded Research
Funding – and where it came from – is always important. This was an all-patient organization-funded study. The funders included WE&ME Foundation, the Poly-Bio Research Foundation, and the Carol L. Sirot Foundation.
And how about the WE&ME Foundation! Funded by Strock family (and their bakeries) in Vienna, the Foundation was created in 2020 after two brothers, Christoph and Philipp, came down with ME/CFS. Over the past couple of years, they’ve helped to fund twelve studies, including two by Maureen Hanson and a couple by Akiko Iwasaki’s group. That doesn’t include their recent call for studies in which they and the WWTF are funding seven studies up to about $100,000 each. Nice!
For its part, the Carol Sirot Foundation gave Akiko Iwasaki $1,000,000 to find a biomarker in ME/CFS. Carol Sirot has ME/CFS.
Conclusion
It was great to see a cluster so prominently pop out in ME/CFS. Time will tell if it was able to capture a craniocervical instability/spinal subset in ME/CFS. (How nice it would be to attempt to correlate these findings with brain imaging results.) Hopefully, this study will lead to a greater focus on connective tissue problems, MMPs, fractalkine, eotaxin, cerebrospinal instability, and other spinal issues, as well as ways to combat connective tissue damage. Speaking of that…
- Coming up – A way to tame connective tissue problems in ME/CFS, FM, and long COVID?





I like spinal fluid studies. But what happened with this study from 2011?
Distinct Cerebrospinal Fluid Proteomes Differentiate Post-Treatment Lyme Disease from Chronic Fatigue Syndrome.
https://www.sciencedaily.com/releases/2011/02/110223171235.htm