

Geoff’s Narrations
The GIST

Could Bezisterim get at core inflammatory processes in long COVID (and similar diseases)?
There are “clinical trials”, “Clinical Trials”, and “CLINICAL TRIALS”. We in the ME/CFS world are used to small “clinical trials” that contain maybe a couple of dozen people, may or may not be placebo-controlled and randomized or not, etc., and are too small to tell us much but can spark interest and can lead to larger trials.
Then there are “Clinical Trials”: large, juicy trials that contain hundreds of people, are placebo-controlled, etc. but are not big enough to be definitive. Then there are “CLINICAL TRIALS”, which can contain thousands of people and are immediate standard setters.
The GIST
-
Bezisterim seeks to intervene early in the inflammatory process
The Bezisterim ADDRESS-LC Clinical Trial that Solve M.E.’s recent webinar focused on is a big, juicy trial that is going to tell us much.
- Biovie, the drug company producing Bezisterim, glommed onto long COVID because the inflammation looked so much like what it had seen in Parkinson’s and Alzheimer’s disease. The trial leader, Biovie’s Dr. Penny Markham, has a close relative with longstanding ME/CFS.
- The trial, funded by the Department of Defense through the Congressionally Directed Medical Research Program, stands out in its expense ($13 million), its large size (over 200 people), and its rigor, including a 4-week pretrial period in which participants will be assessed to ensure they have long COVID.
- Plus, the Bezisterim trial may be the first time a drug company is testing a new (non-FDA-approved) drug in a major trial. Dr. Michael Peluso of UCSF believes the trial will be a trailblazer for long COVID – opening the way for other similar studies to take place.
- Bezisterim is unusual in that it seeks to turn off inflammation by interrupting very basic inflammatory pathways. Because the drug aims to regulate the immune system, not suppress it, it produces few side effects. Its ability to pass through the blood-brain barrier potentially allows it to reduce neuroinflammation. Plus, given recent study findings, its ability to stabilize insulin may be helpful in diseases like long COVID, ME/CFS, and fibromyalgia.
- Plus the trial will measure 400 biomarkers of inflammation/immunity, biomarkers of neurodegeneration, metabolic dysfunction, and DNA methylation (epigenetics) to learn more about long COVID and to find a “signal” that tells Biovie who will benefit.
- It can be a little hard watching long COVID get the big bucks while ME/CFS continues to get slim pickings, but the amount of money pouring into long COVID simply dwarfs that provided for ME/CFS. The way is long for the ME/CFS community, but everything we know suggests that advances in long COVID will ultimately benefit ME/CFS as well.
- Long-COVID patients between the ages of 18-64 with cognitive and fatigue problems who have had long COVID for less than 2 years can participate. Participants cannot have had ME/CFS/FM before getting long COVID. You cannot take LDN for a month before the trial.
- This is a “phase II” trial. If it’s successful, the company will move on to Phase III trials and attempt to become the first drug to get FDA approval for long COVID.
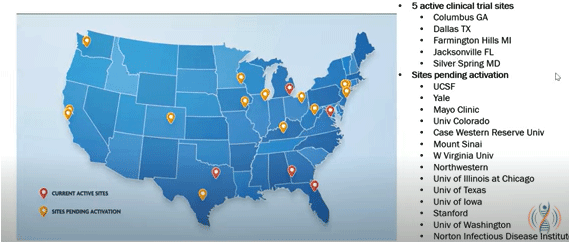
- Five trial sites are open now, but 19 trial sites will ultimately be available. The trial is moving fast and is expected to be complete by early 2026. Find out how to participate here.
The Bezisterim ADDRESS-LC Clinical Trial that Solve M.E.’s recent webinar focused on is the second. It’s a big, rigorously done trial that won’t give us the final word on this very intriguing drug, but if successful, it will open the door to the next trial and tell us quite a bit about long COVID in the process.
Solve ME/CFS Initiative President and CEO Emily Taylor talked with Dr. Penny Markham, the leader of the trial and a Senior Vice President at Biovie, the drug company which makes the drug; Dr. Michael Peluso, the co-creator of the LIINC project at UCSF and a very active long-COVID researcher; and Ezra Spier, a person with long COVID and member of the Patient-Led Research Collaborative.
Dr. Markham said Biovie started thinking about and working on long COVID around 2022. Markham, who has a family member with a 30-year case of ME/CFS, said that when Biovie realized that the inflammatory footprint of long COVID jived with what Bezisterim can do, it applied for funding to the RECOVER Initiative (but was denied). After pivoting to the CDMRP (Congressionally Directed Medical Research Program) – which is committed to funding high-risk/high-reward studies – the trial was funded by the Department of Defense for a cool $13 million.
(While it wasn’t highlighted in the webinar, this trial likely wouldn’t have happened without ME/CFS and long-COVID advocacy. Several years ago, Solve M.E. worked to get ME/CFS included in the Congressionally Directed Medical Research Program (CDMRP) which is committed to “high risk/high reward” research that the NIH, for instance, might not support.
Since that time, several million dollars in ME/CFS grants have been awarded. Then two years ago, ME/CFS advocates got ME/CFS and long COVID singled out as “high-priority” diseases, giving them first choice at the trough. That designation helped Biovie win its $13 million grant for a clinical trial. (That equals the entire funding the ME/CFS field gets from the NIH yearly.) )
The Trial
First, Ezra Speir, who knows the clinical trial long-COVID world, laid out how patients were involved in producing the trial, and why he’s so excited about this particular one. Indeed, while a large drug trial that addresses one of these diseases is noteworthy, the ADDRESS-LC trial stands out in a number of ways.
Dr. Peluso said that for the last five years, it felt like they were “banging their heads against the wall” in the long -COVID clinical trials sphere, but that seems to be changing. As he noted in a recent Health Rising interview, he believes the “first generation” of long-COVID trials has opened the door for a 2nd generation of trials, which features more drug company involvement and better trial designs.
Dr. Peluso felt, though, that the most important thing about this trial is that it’s being done at all. The new drug, the public/private funding partnership, the rigor of the trial, the exploratory measures, and the expense ($13 million-plus!) are showing that the long-COVID field has legs and has matured as a field. He expects the Bezisterim trial to lay the groundwork for similar trials to come.
Bezisterim
Indeed, the ADDRESS LC trial is not your usual long-COVID drug trial. Peluso noted Bezisterim is not a repurposed drug (the RECOVER Initiative is only studying repurposed drugs) but is a brand-new, yet to be FDA-approved drug, and an exciting one at that. This may be the first time that a drug company is taking a chance on testing a new, not yet approved drug in a major long-COVID trial.
Bezisterim seems a drug apart. It seeks to dig so deeply into our biology that it doesn’t fit common drug categories. Listed as an “anti-inflammatory and insulin sensitizing drug,” it potentially has such a wide-ranging effect that it fits into a number of drug categories, including anti-dementia, antiepileptic, antirheumatic, antineoplastic, antihyperglycaemic, antiparkinsonians, antiviral, and nootropic.
Plus, Bezisterim is attempting to get at something more fundamental than most immune drugs. It attempts to turn off (or tamp down) the TLR-4 inflammatory pathway which has been implicated in long COVID and ME/CFS. It’s ability to get through the blood brain barrier means it has the potential to impact neuroinflammation. Plus, its mission – to reregulate immune functioning (instead of to merely suppress it or increase it) – means it produces few side effects.
Dr. Markham explained how the spike protein of the coronavirus can turn on the TLR-4 pathway, but some evidence suggests this pathway may also be turned on in ME/CFS. The effectiveness of low-dose naltrexone – another TLR-4 inhibitor – provides another reason to think this drug might work in ME/CFS and fibromyalgia.
By taming the TLR-4 pathway, Bezisterim is able to calm broad inflammatory pathways such as ERK and NF-kB, which produce an array of inflammatory products (IL-1, IL-6, IL-8, TNF-α).
A long-lasting metabolic derivative of DHEA, Bezisterim is not only anti-inflammatory but is also “insulin-sensitizing”, and that’s potentially a big deal for ME/CFS, fibromyalgia, and long-COVID patients.
A UK Biobank study that examined more than 3,000 blood-based biomarkers found evidence of chronic inflammation, insulin resistance, and liver disease in ME/CFS. Likewise, studies suggest that insulin resistance may be common in fibromyalgia and long COVID. Peter Attia, MD has convincingly shown that problems with glucose regulation and insulin resistance underlie many major chronic illnesses.
Alzheimer’s / Parkinson’s Drug?
Biovie clearly has high hopes for Bezisterim as they chose for their first exploratory trials two of the knottiest diseases of all – Parkinson’s and Alzheimer’s. Their small, 7-month, randomized, double-blind, placebo-controlled trial of people at mild/moderate risk of Alzheimer’s delved deep into Bezisterim’s effect on biology.
While the small Alzheimer’s trial was not successful in significantly moving the needle on cognitive performance, improvements were seen, and Bezisterim was able to improve measures of metabolism, oxidative stress, inflammation, aging, and dementia. Gene expression studies suggested that Bezisterim was turning back the clock on many markers associated with Alzheimer’s, including neurodegeneration (lowered amyloid levels), biological aging, oxidative stress, dementia, metabolic, and inflammation. Plus, in Parkinson’s, the drug was able to improve fatigue/energy.
The ADDRESS LC Study
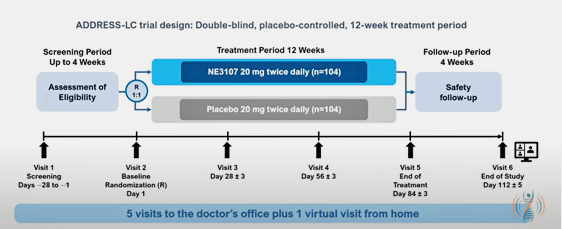
Bezisterim study design.
The ADDRESS LC trial is also uncommonly rigorous. The 12-week, 208-person study includes a 4-week screening period (to rule out other causes of cognitive problems or fatigue) and a 4-week follow-up period. Concerning symptoms, the trial is focusing on cognitive problems that have been validated in long COVID, and is assessing PEM and other symptoms, including a specific focus on tinnitus (:)).
Then there are the “exploratory measures” including over 400 biomarkers of inflammation/immunity, biomarkers of neurodegeneration, metabolic dysfunction, and DNA methylation (epigenetics). Biovie is assessing to understand the biological effects the drug is having, learn more about long COVID, and find a “signal” it can use to identify who benefits from the drug.
Now that’s a clinical trial!
An ME/CFS Note

Yes, the way is long for people with ME/CFS! It seems inevitable, though, that progress with long COVID will move ME/CFS forward as well.
It can be a little hard watching long COVID get the big bucks while ME/CFS continues to get slim pickings, but that’s where we’re at.
In the webinar, Ezra Spier reported how dismayed he was when he learned that his 3-year case of long COVID meant he couldn’t participate. I’m sure he can imagine how people with 10, 20, or more years of ME/CFS feel as they see the many long COVID treatment trials going on…without them.
There’s no denying that the way has been and is long for ME/CFS. Not taking into account the hundreds of millions of dollars a year long COVID is getting via the Congressional authorization to produce the RECOVER Initiative, the NIH, on its own, is spending over $100 million/year on long COVID. (It’s spending $13 million/year on ME/CFS.)
Good or bad, right or wrong, long COVID is where the money is at – and thank god that at least, it’s getting good funding. We must remember that every step forward for long COVID is also a step forward for ME/CFS. Every big, long COVID study or trial is a potential benefit for this disease.. Virtually every long COVID researcher says they have post-infectious diseases like ME/CFS on their minds as well.
If the long-COVID Bezisterim trial works out, the company plans to move to ME/CFS. After all, if the inflammation in long COVID is close enough to Parkinson’s/Alzheimer’s for Biovie to make the jump into long COVID, how much easier will it be for it to make the jump into ME/CFS.
Participating in the ADDRESS-LC Trial
Long-COVID patients between the ages of 18-64 with cognitive and fatigue problems who have had long COVID for less than two years can participate. Participants cannot have had a prior case of ME/CFS/FM. Participants cannot take LDN for a month before the trial or during the 12-week trial.
Unlike other clinical trials, Biovie is not letting the grass grow under its feet. There are no five-year waits for this study – it is moving with lightning speed. The trial has already begun, data collection is expected to be complete in November of this year, and the trial is expected to wind up in Jan 2026 (!).
This is a Phase II trial to assess efficacy and safety. If the trial is successful, Phase III trials to get FDA approval for the drug will begin.
Nineteen sites are planned (five are active right now). If you live near a site that hasn’t been activated, give it a bit of time, and the contact information will be posted.
Want to be part of the study?
Find out how to sign up here.
Support Health Rising! Keep the Information Flowing 🙂
Health Rising is not a 501 (c3) non-profit.







Two big problems which bias the trial towards appearing to work when it actually doesn’t. The first is the 4 week followup, it needs to be a lot longer than that, at least 6 months, we need to ensure relapse doesn’t occur.
The second thing is trying to select patients who are more likely to just recovery with the less than 2 years requirement. There is no reason to do that and its a form of bias that is common. I also suspect they are going to require people walk into their clinic and not defend them from reinfection, further biasing towards mild patients and risking the trial results with noise from reinfections.
Despite their claims about learning from early trials it sounds like its the same errors we have been watching for years.
Interesting point about the possibility of a spontaneous recovery from people with shorter cases of long COVID. I hadn’t thought of that. If I remember correctly, after two years recoveries become rarer and rare. I guess they had to weigh that against the increasing likelihood of more things going wrong over time (???). As I remember clinic visits are going to be a part of the trial – maybe Ezra can chime in here – and home visits will take place as well.
They are learning, though. They’re being more careful about the participants in the study (4 week pretrial period – outside of the intramural study, I’ve never seen that before), and (my favorite) doing lots of exploratory studies alongside the trial.
Very good points, Paul. A couple of thoughts.
Based on its mechanism of action (blocking an inflammatory pathway), I wouldn’t expect this drug to be curative. It might have some long-term benefit but in general I would only expect it to be effective while taking it. I believe that may be why there is only a 4-week follow-up: the expectation is that it’s likely for people not to recover (or to be in remission), once the drug is stopped.
Even though I understand why the researchers have chosen this path, I share your concerns about the 2-year cutoff. I think the hope is that if the drug is found effective in this initial trial, the next trial would have less strict criteria. Also, myself and the rest of the patient reps have been very clear that participants need to avoid infection and we have been assured that study site staff are instructed to be wearing masks at all time. We’ll see how that works in practice, but I’m glad that this is part of the protocol.
Good points, Paul. If you like, write to the study authors; some authors are receptive to and even appreciate feedback.
Do you reckon the 4-week follow up period might have been chosen for a reason, such as to separate the effect of the medication from possible relapse due to other external factors that could happen within 6 months? (They could happen within 4 weeks too though, so maybe it’d make sense at least for the participants with exertion intolerance to ask if external factors have since caused deterioration).,.
“In the webinar, Ezra Spier reported how dismayed he was when he learned that his 3-year case of long COVID meant he couldn’t participate. I’m sure he can imagine how people with 10, 20, or more years of ME/CFS feel when they are shut out of the many long COVID treatment trials going on.”
I sure can, Cort – the lack of trials for ME/CFS is infuriating to me.
Thanks so much for covering the trial.
Ezra! You did such a great job at the webinar! I’m sorry I didn’t get more of your presentation in the blog. Thanks for commenting – and thanks for your support:)
This is really exciting. Do we know any details as to how this drug is or isn’t different from LDN? Would LDN non-responders be expected to benefit?
Great question.
It turns out that they do it in very different ways. Bezisterim turns down TLR4 by phosphorylation (removal of a phosphate group) from ERK (an extracellular signal-regulated kinase). That, in turn, inactivates a key transcription factor called NF-kB which tells genes to start producing pro-inflammatory cytokines. Because it’s specifically targeting them, Bezisterim may be a more potent dysregulator of ERK- NF-kB inflammatory pathway.
On the other hand, by binding to TLR4, LDN blocks it from being activated. That then reduces NF-kB activity and broadly reduces pro-inflammatory cytokine levels.
Importantly, both these can do this in the brain.
Because different mechanisms are used non-responders might benefit. AI Perplexity suggests that “people whose inflammatory signaling is driven by pathways beyond initial TLR4 activation (such as downstream ERK/NF-κB or metabolic dysfunction), or where LDN’s effect is not sufficient to suppress the whole cascade” could benefit.
Because Bezisterim is able to effect metabolism, protect against neurodegenerative effects and even perhaps age-related processes, it may end up being more powerful than LDN.
Time will tell!
Cort, sounds like this drug would be of great benefit in the immediate post viral phase, for example, after the acute EBV period. My intuition tells me this is the phase that, with intervention, could prevent ME/CFS.
I am pretty cynical about much research and trials in the long covid and ME/CFS fields but I think this is genuinely interesting, even exciting.
Fingers crossed
Black seed oil is one of the best natural anti inflammatory
Another crime being committed in Can Can land 🇨🇦
https://globalnews.ca/news/11247648/covid-vaccine-injury-program-visp-oxaro-workplace-phac-2/
I was pleased to see that these clinical studies are funded by the Department of Defense. They have plenty of money and the motivation to find an approved treatment for Long Covid in the military population.
On the other hand, clinical trials can take forever and phase three trials are where the rubber meets the road and many treatments don’t get past this stage.
Skip Pridgen started his work on a combination treatment for ME/CFS in 2013 and the trials aren’t finished yet. Hopefully the DOD has the clout to push things along faster.
What a slog Prigden has had to go through – and while its a new combination – these aren’t even new drugs. The system is truly broken.
I was so surprised to see this phase II trial end so quickly! Phase III trials are bigger and more complex, of course, but this company seems to be able to move things along quickly. Let’s hope the trial is successful and the CDMRP – which advocacy really made possible for ME/CFS and long COVID – does a quick followup and gets things going quickly.
While I appreciate the “Plug” our original research was for fibromyalgia (FM). MECFS patients quickly began requesting that I apply my protocol to them as a patient of mine. We learned that 75% of MECFS patients who had substantial GI issues (that got fixed), and substantial pain components, responded quite well to our combination treatment. The pandemic screwed up Virios’s 35M FM study and Dogwood has no activity on this front now due to limited money. I am excited to report that I was able to make some adjustments to the protocols, have formed a new biotech company -PridCor Therapeutics. We will raise the necessary funds privately to hopefully begin a new patented combo regimen Phase 2 Randomized Double Blinded Placebo controlled 150 person 12 week Long COVID study. Our Pilot study showed an 88% average reduction of Fatigue, dysautonomia, brain fog, & 5 additional symptoms across the board when compared to the combination of valacyclovir and celecoxib alone. Our data extends out 720 days. Me and my coauthors expect to submit this research this week. We hope to begin our new study NLT Q1 ’26.
Thank you, Dr. Pridgen, for your tenacity and commitment to our community.
Thank you for giving us hope.
Thank you Dr. Pridgen, In addition to my LDN which I started in Sept 2024, I have been on the original protocol with acyclovir and Celebrex as prescribed by my Dr. in California since January 2025 while being bedbound with extremely severe ME/CFS triggered by Long Covid. The Pridgen has been a life saver for me – like a switch that eliminated devastating pain. 5 months later I am now vertical, walking unassisted, driving and engaged with the world. THANK YOU for giving me my life back. I hope to be able to continue it. I am forever grateful to the researchers who are working so diligently and swiftly to support us within this community, as champion of patient advocacy we thank you.
So happy that you are thriving again. Email me anytime if questions.
Tsasurgery@gmail.com
Issie had significant side effects (depression) with LDN (TLR4 antagonist) and had to stop it, even if it helped quite well with pain. She had similar side effects with herbals with partial TLR4 effects. I tried several of these herbals and had side effects too (apathy), even if it helped with sleep.
The problem seems to be that there is quite an overlap between TLR4 receptor activation/inhibition and dopamine receptor activation/inhibition. Most chemicals inhibiting one also inhibit the other.
To make matters even more complex, dopamine has a role in the immune system too. At low dose, it seems to protect the brain against inflammatory damage (by inhibiting part of the immune system?). At higher dose, it gets quickly toxic to the brain. Maybe that is why LDN (low dosing of Naltrexone, an TLR4 inhibitor) seems to work best in FM and ME/CFS. Then maybe the optimal point of a bit of TLR4 inhibition together with trying to keep dopamine in an optimal range can be found in a subgroup of patients?
=> Maybe (if not included in the trial yet) the effect on dopamine receptors should be monitored and more importantly LDN style low (and tapered) dosing should be considered in the trial if it is not too late yet.
My memory failed me. Plenty of TLR4 antagonist are also opioid receptor antagonists (such as Naltrexone itself). Those receptors work on endorphins. There is some overlap between opioid receptors and those for dopamine on one hand and oxytocin on the other however too.
Perhaps Ezra can share this additional tracking idea with the researchers.
Thanks Ezra for being the patient rep on this trailblazing study.
Note: I am so excited about this study. But I do find it confusing why they are studying the exact patient population that sees the most instances of untreated/spontaneous recovery. I’m always telling new LC patients to keep hopeful because there is a very good chance they will recover on their own (if they truly rest rest rest) within the first 2 years, post infection. Why didn’t the researchers of this trial select participants who have been sick longer and are less likely to simply recover on their own?
I haven’t heard this, Rivka – can you please elaborate upon which patient population “sees the most instances of untreated/spontaneous recovery”, and perhaps a citation or source of additional details? Much appreciated!
Rivka, very good point on spontaneous recovery. The 2-year requirement is addressed by the lead researcher in the recording of the Solve webinar – here is a direct link to the question: https://youtu.be/-mMgzoZikys?si=Sp5VmnY35VRAX5-c&t=3691
Here’s a quote (slightly paraphrased for clarity): “That’s a hard decision that we made. We are trying to do everything possible in this first study to not miss a signal and we believe that ongoing neuroinflammation in those first two years can be more readily impacted and see a potential impact on cognition and fatigue. Of course if we see this signal, we will be opening up and analyzing our data and, assuming there’s funding, opening as quickly as possible and broadening those criteria. I know it’s restrictive, we’re not enrolling a broad population, but it’s just this first study hopefully of more to come.”
Basically, my understanding is that the researchers believe that the drug is more likely to work for people early in their illness, so despite the potential variable of spontaneous recovery, they still believe that they are more likely to find a signal in that population.
I think the 2-year requirement is the most common feedback the team has received, and from my experience working with them I know they are open to feedback. So let’s hope that they take that into account as the study enrolls.
Thank you, Ezra. Again, they’re lucky to have you working on this trial with them
I do understand the need to have a trial with a positive outcome. If you don’t have a positive outcome with the first trial, then they’ll be no funding for subsequent trials. So researchers really do need to select a patient cohort that they think will be the most likely to respond to the intervention
Metformin and GLP-1 inhibitors are insulin sensitizing and anti-inflammatory. I mean, insulin resistance is inherently inflammatory, right?
In case you think Covid is now a thing of the past, our son and his fiancee are returning from a cruise to Norway. He just called and said a member of their travelling group has Covid.
From what I can tell, the symptoms are the same as the Covid I got in 2022 that has caused Long Covid.
Be careful especially if you are traveling or will be in a large group.
I’m very interested have great qualifications as a Lab Rat
Once again, this drug is seeking to eliminate a symptom…inflammation.
“Inflammation is the body’s way of defending itself against harmful stimuli like pathogens, damaged cells, or irritants, and it’s a crucial part of the immune system’s response. It essentially helps the body eliminate the initial cause of injury, clear out damaged tissue, and initiate healing. Inflammation is a vital process for maintaining tissue homeostasis and adapting to adverse conditions. ”
We need to find out why the inflammation is happening, in response to what?
You’re absolutely right, Betty. First of all, it’s not at all certain whether there’s a brain infection/inflammation. Not in ME and not in long COVID. Furthermore, it’s not proven whether, and if there is, inflammation, it’s mediated by the ERT or NFkB mechanisms. This drug still needs FDA approval. The fact that the manufacturer believes this drug could be effective for many diseases, particularly in the brain, seems like a red flag to me. I don’t have high hopes for it.
Exciting stuff, thanks Cort. This dovetails very nicely with Dr Jarred Younger’s very recent announcement of PET scans showing inflammation throughout multiple regions of the brain.
I hope Biovie and Dr Younger will be able to join forces and establish bezisterim’s impact on brain inflammation, thanks to its ability to cross the blood-brain barrier.
I also expect that, if it receives approval first for a condition like Alzheimer’s, physicians and patients will be using it off-label for conditions like Long COVID and ME/CFS.
I didn’t know Younger has found inflammation till yesterday. If that is true this drug could be helpful. But still we need to know what causes the inflammation.
I think it’s pretty clear what causes the inflammation in a large number of cases: an immune system that’s gone into overdrive because of an infectious threat that occurred in the past, whether it was COVID, candida, Epstein Barr, chemical weapons, mold, or what have you. Even if the threat is long gone, the traumatized and hyper-vigilant immune system continues spitting out cytokines and other substances that keep the brain in a constant state of arousal.
It’s like a dog that was abused at one point and now sees every human as a threat. And thus the inflammation is unnecessary and in fact counter-productive, and dampening it will likely not be harmful.
Cort !
anyone interested in nicotine as a therapeutic for CFS
should read this paper to encourage them:
https://www.sciencedirect.com/science/article/pii/S0002944012003380
I have believed for some time that a7 Nicotinic Acetylcholine receptors are central in CFS.
These a7 receptors are present:
system-wide in blood vessel endothelial cells
on the epithelial tight-junction cells of the intestine
in the BBB of brain blood vessels
at skeletal muscle (long-term potentiatiin needed)
at cognitive brain synapses for learning and memory (long-term potentiatiin needed)
in the iris of the eyes
in microglial cells of the brain where they can launch anti-inflammatory measures
in the hippocampus specifically
TRPM3 ionic llgand-gated receptors ARE a7, as are the ACh receptors for skeletal muscle.
in the Vagus nerve, which, as you will see in the linked paper, if stimulated will be anti-inflammatory (ie. stop the fight-flight)
and also will do so with nicotine.
Nicotine was the only natural compound that prevented death in covid by reducing vascular inflammation.
Therefore, this receptor can be blamed for brain fog, fatigue, apoptosis or its failure, hypoxia, POTS, leaky gut, leaky brain, failure of our anti-inflammatory systems, most importantly microglial and Vagus nerve.
And non-cigarette nicotine has the potential to treat
ALL OF THEM.
at cognitive
Oh yes…
and
Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria
so, the a7 might also be blamed for the excess build-up of calcium in CFS cytosol
Just wanted to follow up here and share the news that the trial protocol has been amended to include participants who have been ill for more than two years. It was announced at a conference this week, and here is a press release: https://investors.bioviepharma.com/news/news-details/2025/BioVie-Highlighted-ADDRESS-LC-Phase-2-Trial-Design-Exploring-Bezisterim-for-the-Treatment-of-Neurological-Symptoms-of-Long-COVID-at-Keystone-Symposia-on-Long-COVID-and-Other-Post-Acute-Infection-Syndromes/default.aspx