
Something new! Thanks so much to Geoff for doing a voice narration of the blog :). If you have any problems please let us know.
This is the first of two blogs on atypical thyroid issues in diseases like chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), and long COVID. If you’re taking thyroid and aren’t getting the boost you expect, these blogs might be for you. They’re also for people who’ve never had their thyroid thoroughly checked out. In a recent YouTube talk, Jarred Younger stated that he regularly finds people with undiagnosed thyroid disease, diabetes, etc. in his ME/CFS studies.
Thyroid hormones regulate the metabolic rate of virtually every cell in the body. The thyroid gland, though, is just the beginning of the story.
All you need to understand why the thyroid is of interest to people with these diseases is to read the following sentence from a 2023 German study, “Autoantibodies to selenoprotein P in chronic fatigue syndrome suggest selenium transport impairment and acquired resistance to thyroid hormone“. It reads:
“Thyroid hormone (TH) is a central regulator of temperature, energy metabolism and psychic well-being.”
When you look at the symptoms associated with low thyroid (hypothyroid) (fatigue, increased sensitivity to cold, muscle and joint pain, muscle weakness, irregular menstrual periods, anxiety), you can see why many doctors often turn to the thyroid when fatigued, sleep-deprived patients show up at their door.
When the authors said “central”, they meant really “central” – as in fundamental for most of the cells in our body. It turns out that the production of thyroid hormone (thyroxine – T4) by the thyroid gland is just the beginning of the process. T4 then has to be broken down into its usable form – T3 (triiodothyronine) – and that’s where things get interesting.

THE GIST
- This is the first of at least two blogs on atypical thyroid issues found in ME/CFS, fibromyalgia, and possibly long COVID
- Thyroid hormones are one of the master regulators of the body. The authors of this study stated, “thyroid hormone (TH) is a central regulator of temperature, energy metabolism, and psychic well-being.” When they say central, they mean central: thyroid hormones regulate the metabolic rate of nearly every cell in the body.
- When you look at the symptoms associated with low thyroid (hypothyroid) (fatigue, exercise intolerance, increased sensitivity to cold, muscle and joint pain, muscle weakness, irregular menstrual periods, and anxiety), you can see why many doctors quickly turned to the thyroid when fatigued, sleep-deprived patients showed up at their door.
- The thyroid hormone produced by the thyroid called T4 is just the beginning of the process. T4 is then converted into its usable form – T3 – not in the thyroid, mostly, but in cells in the brain, liver, kidneys, muscles, etc. Selenium-based enzymes play a critical role in transporting T4 into the cells to be converted into T3.
- Since the thyroid gland mainly produces T4, thyroid supplementation seems straightforward: simply provide T4 and the system should work. In a significant subset of people getting T4, though, it doesn’t and they remain fatigued, have difficulty exercising, experience muscle and joint pain, etc. Since they’re getting enough T4, it stands to reason that a problem may exist in getting the active form of the thyroid (T3) into their cells.
- A German team of researchers examined whether the selenium-based T4 transporters were getting attacked by autoantibodies in a large number of ME/CFS patients and healthy controls. They found increased levels of selenium autoantibodies in about 10-20% of ME/CFS patients.
- The high autoantibody levels were associated with several findings suggestive of thyroid hormone failure: “particularly low” enzyme activity, low free T3 levels, low total T3 to total T4 (TT3/TT4), and low free T3 to free T4 (FT3/FT4) ratios.
- If you’re not getting the boost you should be getting from thyroid supplementation, these increased autoantibody levels could be why. The senior study author proposed this scenario would cause, “hypothyroidism in muscle, with poor activation of the mitochondria and consequently low ATP levels (explaining fatigue and PEM).”
- Further study is needed, but the authors suggested that selenium supplements plus pure T3 could be helpful. The senior study author stated, “Se supplementation is safe within the limits known from many intervention studies, i.e., up to 300 micrograms extra per day.”
- Because T3 – the pure, active form of thyroid – is so potent, using it is trickier. The author stated, “With respect to T3, as with all thyroid hormone treatments, it is a very personal and individualized issue. One does need to increase the dosage slowly, titrating it, under monitoring TSH levels, and most importantly, monitoring the patient’s well-being… We assume that in particular, those hypothyroid patients with impaired expression of Se-dependent deiodinases would respond favorably to the T3/T4 combinations, as their activation of T4 to T3 in the peripheral tissues is compromised.”
- A test for selenium autoantibodies is available in Germany but not yet in other countries.
- Next up – exploring another atypical thyroid condition in ME/CFS, FM and long COVID – low T3 syndrome.
Once in the cell, T3 regulates the metabolic rate of nearly every cell in the body. It does this by increasing the gene expression of Na+/K+ ATPase enzyme, resulting in increased oxygen consumption, energy production, and body temperature. T3 affects muscle control and brain, heart, and digestive functioning. In short, it’s one of the master regulators of the body. (To think that it all starts in a little butterfly-shaped gland in the front of our neck.)
Given the exercise intolerance in ME/CFS, it’s intriguing that thyroid problems (either hypo or hyperthyroidism) can also cause exercise intolerance. Reduced workloads and heart rates at the anaerobic threshold have been found.
It’s no wonder that thyroid problems are one of the first options doctors turn to when fatigued, exercise-intolerant ME/CFS/FM and long-COVID patients show up.
The Crucial Conversion
The inactive form of the thyroid hormone (T4) is converted to the active form (T3) by 3 selenium-based enzymes (isozymes: DIO1, DIO2, DIO3).
- DIO1 and DIO convert T4 to active T3
- DIO3 converts T4 to inactive reverse-T3 (rT3), and T3 to T2
Because these isozymes or enzymes rely on selenium to function properly, a selenium deficiency can result in thyroid problems and affect iodine excretion.
The Hashimoto Disease Connection
The paper’s authors first worked on a problem found in some Hashimoto’s syndrome patients. Because Hashimoto’s disease involves the destruction of the thyroid gland, it would seem to be amenable to a simple fix – simply supplement with the main end product of the thyroid gland – T4.
The T4 supplementation doesn’t always work, however – some Hashimoto’s patients remain fatigued and impaired. Hashimoto’s Disease, it should be noted, is not the only disease in which thyroid supplementation can be a bust. We’ll see in the next blog it’s not uncommon for people on thyroid medication to feel not up to par.
Since thyroid supplementation brought the T4 levels up to normal, it appeared that something was interfering with the conversion of T4 to T3.
Selenium-Based Enzymes Take a Beating
Looking for an answer, Schomburg’s German group dug into the selenium-based isoenzymes that break T4 down. Because the thyroid isozymes are only found within the cells, and can’t be readily studied, they looked for signs of selenium issues elsewhere, and landed on testing another selenium-based enzyme called glutathione peroxidase (GPx3).
Low levels of glutathione peroxidase (GPx3) in the poor T4 responders with Hashimoto’s suggested selenium was not getting into their GPx3 enzymes either.
The Autoantibody Connection
The next step was to see if autoantibodies that might be inhibiting selenium entry to the cells were present – and they were: increased levels of autoantibodies (SELENOP-aAb) to the selenium transporter (selenoprotein P) were found.

Autoantibodies attacking selenium-based enzymes provided the crucial link. (Image from Wikimedia Commons)
With that, they had indirect validation of their hypothesis: the Hashimoto’s patients who weren’t responding to the standard thyroid hormone (T4 – levothyroxine) because their cells weren’t converting T4 to T3 as they should.
The T4/T3 conversion was blocked by selenium autoantibodies that were preventing selenium from entering the cells. That left the selenium-based isozymes needed to convert T4-T3 in the cell high and dry.
The ME/CFS Study
With that, they turned to chronic fatigue syndrome (ME/CFS) and tested selenium levels, selenium autoantibodies (SELENOP-aAb), and glutathione peroxidase (GPx3) in a lot of patients (n=167) and healthy controls (n = 545).
They found greatly increased levels of SELENOP-aAb in a subset of ME/CFS patients and hardly any healthy controls (9.6–15.6% in ME/CFS patients versus 0.9–2.0% of healthy controls, depending on what the cutoff for a positive result was). They reported that the “prevalence of SELENOP-aAb in CFS was about 10 times elevated in comparison to controls” and exceeded that reported in thyroid or cancer patients.
They dug deeper. Because selenium plays a crucial role in the glutathione peroxidase enzyme, selenium levels should be correlated with glutathione peroxidase levels; i.e. higher selenium levels should be correlated with higher glutathione peroxidase levels. The fact that they were not in ME/CFS suggested that the autoantibodies were preventing the selenium from being used properly elsewhere in the body.
That’s an interesting finding given that glutathione is the major antioxidant in the body and high levels of oxidative stress have repeatedly been found. Because glutathione peroxidase is the main antioxidant in the body, it was not surprising to see elevated levels of nasty free radicals/reactive oxidative stress species called isoprostanes in the urine. That finding jived with several studies in the mid to late 2000s from UK researchers which found increased levels of isoprostanes in ME/CFS.
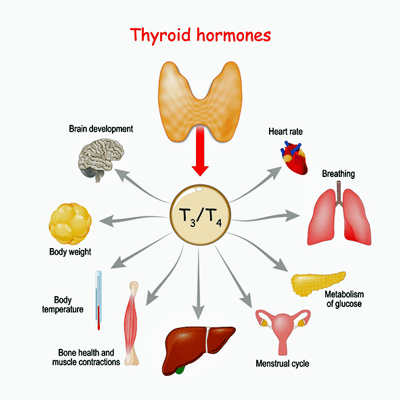
Thyroid hormones affect the functioning of many systems in the body.
Plus, the high Se autoantibody group stood out in a group of ME/CFS patients and healthy controls who had undergone extensive thyroid testing. The high Se autoantibody group exhibited “particularly low” DIOD isoenzyme activity, low free T3 levels, low total T3 to total T4 (TT3/TT4) and low free T3 to free T4 (FT3/FT4) ratios.
Those findings suggested T4 was not being properly converted to the usable form of the thyroid hormone (T3) in this group.
Going further, they also found low urinary iodine levels in this group. Because iodine is released from T4 when it is converted to T3, the low urinary iodine levels suggested (again) that T4 was not being broken down properly.
The authors proposed that ME/CFS clinical trials involving selenium and T3 take place.
The Schomburg Interview
I asked the senior study author, Lutz Schomburg, how he got involved in this study, what safe levels of selenium might be, and what’s next for him and his crew. Schomburg is Dept. Director of the Institute of Experimental Endocrinology at Charité Universitätsmedizin Berlin. Schomburg wrote:
“Once I had the honor to get into personal contact with some CFS patients and learn about the devastating nature of the disease, myself and my full research team became highly motivated to dedicate a lot of our efforts towards this topic, as it also nicely falls into our primary research issue of nutrition, autoimmunity and thyroid hormone research.”
He said he was convinced that this selenium autoantibody – thyroid interaction is present in 10-20% of ME/CFS patients.
“I am convinced that the link between impaired Se transport and ME/CFS is real and operative in a subset of patients (10-20%). We are currently testing the hypothesis that it may even be a general mechanism, also of relevance for tumor (post-cancer) fatigue or long-COVID.”
Concerning selenium supplementation, Schomburg said this:
“Se supplementation is safe within the limits known from many intervention studies, i.e., up to 300 micrograms extra per day. There is a slight risk of side-effects (type 2 diabetes) for overweight males residing in Se-rich areas like the USA, but there is no indications for any side effects for women or subjects residing in Se-poor areas like Europe.”
Several studies suggest that supplementing selenium with myo-inositol can improve thyroid function. (Reduced selenium levels have been associated with an increased risk of Hashimotos Disease.)
T3 is a more complicated subject:
“With respect to T3, as with all thyroid hormone treatments, it is a very personal and individualized issue. One does need to increase the dosage slowly, titrating it, under monitoring TSH levels and most importantly, monitoring the patient’s well-being.”
“Thyroid hormones in excess carry the risk of tachycardia and arrhythmia – that is why usually the prohormone T4 and not the active hormone T3 is prescribed and supplemented. But recent research has indicated that many (especially female Hashimoto’s) patients do profit from a T3/T4 combination, like the normal secretion pattern from the thyroid gland and like found in dried thyroid extracts (at a ration of around 1/10th of T3 compared to T4).”
“We assume that, in particular, those hypothyroid patients with impaired expression of Se-dependent deiodinases would respond favorably to the T3/T4 combinations, as their activation of T4 to T3 in the peripheral tissues is compromised.”
Schomburg then spelled out how low T3 problems could impair muscle activity and produce exercise intolerance.
“If you imagine muscle as target tissue, impaired T4 to T3 conversion would mean hypothyroidism in muscle, with poor activation of the mitochondria and consequently low ATP levels (explaining fatigue and PEM). The diagnostic problem is that we cannot determine T3 status in muscle, only in the circulation, which of course is not always a reliable mirror of what is going on in the target tissues.”
“Yet, while I am convinced that the patients with impaired Se transport will profit from such Se and T3 treatment, other patients with intact Se status and transport may not, and may even experience cardiovascular side effects from hyperthyroidism.”
“This is why I strongly recommend to test these experimental treatment strategies only in collaboration with the treating physician, and ideally after an analysis of Selenium and Selenoprotein P (SELENOP) concentrations, and an assessment of potential autoimmunity to SELENOP.”
“Unfortunately, these analyses are not (yet) available in the USA, but I am very happy that we have succeeded in convincing a diagnostic provider to offer these analyses in Germany, and hopefully soon also elsewhere. (https://www.biovis.eu/de/).”
Thanks to Sue for the link to this intriguing finding!
- Next up – The Atypical Thyroid Issues in ME/CFS, FM, and long COVID Pt. 2 – Low T3 Syndrome






How can I get a copy of the article? I’ve been telling my doctors for years there is something wrong with my muscles, in addition to my hypothyroid. No one believes me.
I’d like that too, if possible. I8
The full study is available for free at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10338150/
Thank you!
I have exactly the same problem. It’s very hard to convey to a doctort how much this affects your life.
Me tooo! Have been diagnosed with hashimotos, hypothyroidism, and ME/CFS over 20 years ago
I’m glad that finally someone accept a non genetic form of thyroid resistance. We know so little about the action of the thyroid hormones into the cells. Although I don’t understand the interest of selenium supplements if there are anyway antibodies that don’t allow a correct absorption. I always used some selenium supplements periodically and never saw a difference. I deeply believe that ma me sfc condition was a result of some kind of th resistence.
I only read “The Gist.” I take T/3, T/4 with little to no difference. I prefer to be overdosed with it, but my GP won’t allow me to go outside normal lab results. Would it make sense to supplement with Selenium now, or wait for some reason?
Ft4 and Ft3 “normal ranges” with with TSH suppressed? for me to get into mid range “free T3” my TSH will be suppressed. most doctors never like that.
Yes. My TSH gets suppressed if I increase T3/4. My ME/CFS specialist didn’t mind, but my GP did. My specialist is no longer my doctor now and I don’t have a new one yet. I feel much better on higher T3/4 doses.
My consultant actively expects the TSH to be suppressed when on T3 supplementation and had to write to the GP to inform him, as he did not have sufficient knowledge of the subject.
Thank you, Geri. I’ll need to get a new specialist , and as my insurance is changing, a new GP too. But it’s good to get that piece of information.
My endocrinologist says the TSH level is close to meaningless if you’re taking thyroid supplements.
Of course, you can still get a regular blood test and get some idea where your selenium is. It’s one of the few nutrients that you really don’t want to overdo. Also, popular opinion amongst thyroid pages is that your T4 should be in the middle to upper range in your T3 in the upper fourth of the range. Of course, having a high reverse T3 will make much of that T3 unusable so that test always needs to be done too.
Thankyou Cort. I am wondering where “Reverse T3” fits with all of this? The reason is, my thyroid levels are normal, but my “Reverse T3” levels (another often overlooked, additional test) is off the charts high. In fact my integrative GP said they were the highest level he had ever seen!
Reverse T3 dominance:
https://custommedicine.com.au/health-articles/reverse-t3-dominance/
Thanks for the article, Wayne! I also have really high Reverse T3 and my doctor wants to reduce my NP Thyroid and possibly increase my T3 but I was worried about getting even worse. I’ve been on a high dose (185 mcg) of compounded slow release T3 for a while now & my basal temp still runs 94 to 95 degrees. Daytime temp runs 97 to 97.5 degrees. After reading this article, I’m thinking I’ll give it a try. Mine’s not Hashimoto’s.
For those with doctors who are clueless, maybe try educating them on the fact that TSH levels will always be at ~ 0.00 if you’re taking any amount of T3. Before I became hypothyroid, my TSH was normal at 0.4.
Educating a doctor?….geeez, they sit on top of the world.most if not all the Dr’s. I’ve ever met think they are god
“TSH levels will always be at ~ 0.00 if you’re taking any amount of T3.” Not true. I take separate T4 & T3. My last levels were TSH 10, T4 .8, T3 2.2. Taking 70mcg T4 & 30mcg T3. Had to decrease the T3 to 20 as heart palpitations. Hasn’t made any difference in my low body T* or other symptoms.
Sorry, T, was simply repeating what two different specialists had told me over the years. Maybe too are differences in where labs were drawn? In the US a TSH of 10 would be considered to be severely hypothyroid. The reference range on my labs for TSH is 0.300 – 3.000 mcunit/mL. Looking at my labs I see my TSH is not 0.00 but 0.005.
It’s not your fault. Just wanted to let you know someone gave you some wrong info. Why am I not surprised it’s a “specialist”? And yes my thyroid is out of control right now. Started taking 200mg selenium and my TSH from this week is 6.4. Still have a ways to go but at least it’s better. This article helped a lot! Will need to check into all the good info more. And I can see with your scale that in the US zero would actually be a good number. 🙂
Hi Cort.
Hypothyroidism of the muscles????
This question is insanity as worded.
We both know that the
HPA Axis is controlled and instructed by the Hypothalamus,
which has been called : if there were a brain that controlled the brain, the HTHY would be it.
The muscles can NEVER…EVER tell the HPA what to do!!!
If they could, or did… ALL OF YOU would be dead. Not kidding.
The brain directs the body to do what the brain needs it to do.
I stopped reading past the first paragraph because that question is preposterous.
If thw body dose some rogue crap that the brain does not sanction, then the system
SHALL DIE.
The H sets a temperature set point.
Usually it is 98.6 F in healthy states,
but those who rely on metric are saying that 37 C is most accurate.
If you are “insulted” by a pathogen then your H might tell your P to tell your thyroid to convert Th 3 to Th 4
and sustain a fever.
That would be step 1 of an innate immune system preliminary response to a pathogen, until antibodies have been formed … within up to 5 days. Covid might delay it.
So… NO !!!! Muscles dont get a carte blanche for energy production/cutback.
If it were possible: DEAD, gone.
What makes ACTUAL sense is for your H to realize that Pituitary instruction to ramp up energy production in MOTOCHONDRIA, not muscle-specific,
would result in BRAIN FEVER for an already Microglially activated inflamed brain!!!!
😎 I know it sounds crazy – but that’s what the endocrinologist said. Plus, it makes sense – if you read the blog, you’ll see that thyroid hormone is broken down into its active form in the cells. After the active form of thyroid – T4 – is produced by the thyroid. It’s all up to the cells – and if they are not doing their job, it’s entirely possible to have a hypothyroid state in the muscles or other tissues in the body
Thank you Cort for that brief underline of the key reasons that muscle— or even cognitive brain cell hypothyroidism could occur, and limbic cell too.
My reaction was an overreaction, as if you had claimed the world were flat.
I would like to share with you the a/b alternatives that I struggle with each day on this topic:
1. I thought that either the thyroid was not sending hormone, or the mitochondria were unwilling to generate more heat it. It was a draw. But to look at non-shiverring as a FAILURE to use T3, now that is news.
2. As you know, researchers postulate that either the mitichondria have been damaged, or the DNA has responded to Hypothalamus lower temp. set point by choosing an alternative mitochondrial phenotype (configuration) suitable to the occasion. (continued need for inflammation, but a continued need to deprive pathogens of regular ATP levels.
The fact that there is a selenium autoantibody changes things.
There is also in existence an AChR antibody. This is why I brought up Mestinon earlier. Mestinon would keep the ACh at the receptors longer, allowing longer-term muscle or thought potentiation.
I did not know that the selenium was needed in the cell, not the thyroid.
And then we see recurrent (in stats) presence of insulin resistence.
I think this blog mentioned undiagnosed T2 diabetes. Im
scratching my head as to how T2D features insulin resistance, yet diabetes or pre-diabetes is not investigated immediately upon the finding.
If Selenium resistance or destruction is occurring, then I can see why the cell would switch to anaerobic. It would start aerobic but have insufficient selenium for oxygen-based metabolism to continue. The cells CAN do work, but not for long.
This also supports OR IS THE “why??” in the Itaconate shunt hypothesis. Early switch to anaerobic because the action has begun but is not finished. It’s got to finish somehow.
The muscles FEEL they must quit before they actually have to quit.
I think this allows for autonomic behaviour such as quitting exertion and seeking shade in high outdoor temps. You cant very well go to shade if you have fainted.
But that brings up dyslipdemia (HDL/LDL imbalances, impaired cholesterol processing….)
Theory:
Thyroid tells muscle or cognitive cell to work.
Cell works to a point but from either insufficient glycogen reserve from insulin resistance,
Or
from insufficient T3 conversion from selenium deficit,
Cell switches (Rob Phair) to fatty acid metabolism.
Since the mitochondria are either damaged or reconfigured in the CDR Cell Defense Response (Naviaux) , the ATP CREATED shunted and sequestered.
At that point, only protein metabolism can occur.
Lastly: if Selenium is key to glutathione production, which I did not know, this helps to explain why inflammation flares with exertion.
Thanks Christopher for your very interesting comment. I love how you brought the different ways to get to an emphasis on anaerobic energy production. It also makes me think of insulin resistance. Marco wrote an interesting piece on that some time ago.
https://www.healthrising.org/blog/2014/02/26/energy-disorders-diabetes-cfs-fm-can-diabetes-tell-us-chronic-fatigue-syndrome-fibromyalgia/
Crucial distinction needs to be made;
Between Hypothyroidism and Adrenal Fatigue from sustained Hypercortisolism.
Please Read Dr. Lam Coaching in USA:
https://www.drlamcoaching.com/adrenal-fatigue/
It is important to read the whole article.
The second article is just as important.
https://www.drlamcoaching.com/blog/resistance-to-hypothyroidism-medication-and-adrenal-fatigue/
It differentiates body temperature states in Hypothyroidism vs Adrenal Fatigue.
I believe that from chronic infection (low level inflammation)
the CFS patient is in a state of Adrenal Fatigue, not hypothyroid.
The simple distinction is that hypothyroidism gives low body temp and sensitivity to cold 24 hours per day.
But in Adrenal fatigue temperatures rise and fall in an incorrect pattern, leaving highest cortisol at bed time and over-temperature for restorative sleep.
Plus 3-3:30 am premature waking. Then when the patient ACTUALLY gets up for the new day, the cortisol levels fo not reach “I feel awake” levels for up to 2 hours.
That’s me!….I awake at 3am…310am…312am…all the way up to 340….i am litterally wide awake until around 5am-6am.then I will go either watch TV or just lye in bed until I can fall asleep again.
I’ve always thought it was due to something with my spine and the perrin technique etc
….toxins not being able to flow properly. I’ve got a herniated disc (L4-L5) and arthritis in my neck due to being rear ended while driving.
My sister has scoliosis.pretty sure y illness is genetic
Wayne – I had off the charts high levels of reverse T3. She prescribed Adrenal Support by Vital Nutrients beginning with 1 capsule every a.m. I had so much relief and by 3 months had lost nearly 75lbs along with helping tame chronic severe anxiety.
I also have high Reverse T3. It’s 31.I feel extreme anxiety, brain fog, and muscle twitches daily. my vision also goes blurry. I’ve also gained weight and have increased cholesterol. Did you have brain fog?
I have taken T3 only for many years & found it very helpful. I’ve also taken all sorts of combinations of T4 & T3. High Reverse T3 is caused by unused T4 in the body. It’s caused by the body being unable to convert the higher doses often taken in desperation that need to cleared from the body. High reverse T3 in itself isn’t apparently as harmful as once thought but is indicative of problems of non conversion or of other illness. Taking T3 only is the solution & this research is the first I’ve ever seen which explains why conversion doesn’t take place in some people. It’s very interesting & will be useful to the many hypothyroid patients who do poorly on T4.
This is extremely interesting (as are the comments by other commenters above – what a knowledgeable group!).
May I ask what form of T3-only you’re taking? I want to take all this to my current endocrinologist.
Also, do you have to take it every three-four hours or so during the day? That’s what stopped me from following a T3- only regime years ago.
Hi, I take a German brand of T3, Liothyronine, Thybon Henning.
In the UK this can be obtained from prescribing chemists eg Roseways. It’s possible to book online for an appointment with a prescriber. Private blood tests from somewhere like Medichecks would be necessary showing a low level of T3.
Alternatively a few enlightened endocrinologists will prescribe. A list is available by emailing the charity Thyroid Uk, which also has a very active forum where you can post questions.
Once you have T3 medication, which is cheapest as a 20mcg tablet you need to start with a small dose 5mcg & increase slowly. ie 5mcg more every 6 weeks. If you go too quickly you may well overshoot your individual sweet spot.
I did take a dose three times a day, setting an alarm on my phone. T3 needs to be taken away from food & drink which is tricky though it’s less sensitive than T4. Eventually when settled on an appropriate dose people can take it in one go. I tried this but prefer 2 doses, 1 on waking, 1 in the evening.
There’s so much to say I could write for hours but please ask more questions & check out the Thyroid Uk forum.
If you’re having blood tests for thyroid hormones remember ONLY have them fasted, before 9am & 24 hours after last T4 dose & 12 hours after T3 dose for accuracy.
Doctors will often cite reasons for not prescribing T3 as it harms the heart & thins the bones. Both are not true. In fact, the opposite is true. Cardiologists are more likely to use T3 than endocrinologists. It’s crazy.
Thanks, Sue. That’s all I needed to know. Alas, I have a long history with thyroid, testing, etc.
It’s much better to take T3 in divided doses as this mimics the body’s natural rhythms.
Hi, some people, probably with thyroid hormone resistance, find that taking one large dose every 24 hours helps with absorption of the T3. It can be a very personal thing taking T3 only, everybody’s different. Probably one of the reasons it’s considered difficult to use by many doctors. There are no rules about dosing. Watching & noting symptoms, taking temperature & pulse, blood tests all help in determining dose & timing. To echo natural levels a high dose first thing in the morning & then 2 doses through the day is closest.
I have no idea – I have a lot to learn or relearn about thyroid (lol)
Hi Cort, thank you for posting about this research. It’s caused much excitement on the Thyroid Uk forum where many of us relate our battles to be tested properly & then be prescribed T3, Liothyronine.
I recommend it as a great source of thyroid related information & we welcome any questions.
Thanks, Sue! It’s fascinating stuff to be sure. More on thyroid is coming 🙂
This sounds like a very promising direction to go down. Thank you for making it understandable to non scientists like myself.
One of my sons is going to Germany for a few weeks in May. Maybe I could ask him to see if he can get the selenium test for me. Do you think it would be available in Chemists or would it need to be prescribed by a doctor?
I really appreciate all of your updates Cort. It gives me hope for the future.
All the best,
I don’t know but here’s the website for the test – https://www.biovis.eu/de/. Good luck!
Thank you
This looks like a lab test (not at-home test). You could inquire with the lab whether it is possible to send in blood for analysis from abroad. It will likely depend on how long and at what temperatures the blood serum is allowed to be travelling in order to still be good for this test. Contact information can be found in the webpage impressum https://www.biovis.eu/en/impressum/ .
I just emailed the lab to ask how my child’s endocrinologist might work with them to do the 3 selenium tests. I’ll write in again if I get an answer back.
Ok, lab got back to me: can’t work with doctors in the US 🙁
I wonder how many people diagnosed with ME/CFS actually have dystrophic myotonia, myotonia congenita, or acquired myotonia?
Hypothyroidism is very prevalent in myotonia. My son was just diagnosed with DM2 and myotonia congenita via genetic testing and EMG. His doctor mentioned that my son was lucky to receive the correct diagnosis which usually takes 14 years. The doctor stated that many of his myotonia patients had previously been diagnosed with ME/CFS.
My son was also diagnosed heterozygous in the SELENON gene. His selenium runs out of range high in serum and RBC. He has no symptoms of selenium toxicity and avoids foods and supplements with selenium.
Because of no relief from my hypothyroidism and subsequent treatments my doctor recommended I add liposomal liquid iodine. The addition proved very helpful.
I was interested when I first read the study a year ago, but quickly lost interest when I couldn’t decipher some detailed about the cohort they used. Apparently patients were recuited from the Parkstad Clinic in Amsterdam and an unspecified clinic in Valencia, which both don’t seem to be anything of relevance and I was wandering why they weren’t using samples from the Charite Fatigue Clinic situated virtually next door?
Do you have any information on this?
Running this test on samples from the CFC shouldn’t be too hard?
Interesting. I assume they found out about ME/CFS through the work Charite is doing. I remember seeing different cohorts but I didn’t look into that. I think one they used simply because they had a lot of thyroid data on them
This is good information but it isn’t new. I’ve known about this for years. Several other previous studies correlate with these findings.
If you have some links please share them. A search for thyroid autoantibodies chronic fatigue syndrome just pulled up this paper.
Thank you so much for this article, Cort. I’ve been waiting for some validation for my thinking for years. 25 years with severe hypothyroidism before diagnosis, with no benefit from treatment. Another 8 years then ME diagnosed. Test results show low TSH, high T4 and, when I can get it tested, very low T3. Have lived a ‘half life’ for 60 (!) years. Perhaps there is a glimmer of hope that there is some chance of improvement. Looking forward to your next blog re LC and low T3 Syndrome.
Thank you so much Cort for posting this very interesting study, and making it much clearer for the layperson. I am in touch with thyroid groups in the UK who are despairing at the problems with getting endocrinologists to prescribe T3, so I think they will be interested too when I share it with them.
I was diagnosed hypothyroid at aged 44, many years before I had the ME/CFS diagnosis. However despite being given T4 it took a long time for my TSH to normalise. I had just a few years where I felt relatively well and then as my menopause kicked in, my health began to slide slowly downhill until I was finally diagnosed with ME/CFS.
I never tied up the thyroid issues with the ME/CFS for years, and then I began to research, and got myself referred to an endocrinologist in about 2012 who was willing to prescribe me T3 – initially T4/T3 and then T3 monotherapy. This is in the UK, so was unusual then, but now is almost unknown to be on T3 monotherapy for free on the NHS. It was a bit of a battle when the price was so artificially high here for years, and I did have endos emotionally blackmailing me to get off it. This included one who sent me off for a DEXA scan, convinced that my below range TSH would have caused me to have osteoporosis despite no broken bones. The test came back positive for OP, but I have never had a fracture. It took me until last year to disprove this test, via a private one of my own (Osteoscan) which detected the misdiagnosis – I have Osteopenia, which is perfectly normal for someone who is now over 70. I have also never had any atrial fibrillation.
The T3 monotherapy was not a miracle cure, but I have generally felt better on it especially last summer, though after this winter I have gone downhill again, and am trying out red light therapy to see if that will help as the winter in my flat has been dark, and I now know that LED lighting has very little red light in it, so will also be buying a full spectrum lightbulb.
I have also checked my selenium intake, as I used to take more in a supplement and ate brazil nuts more in the past. I see that my supplement dose is now only 100 and I don’t take that every day, and I am in a country where selenium is low in the soil. So I will increase my intake (below 300) and see how I do as the Spring/summer progresses.
Speaking of brazil nuts, do I remember correctly that only brazil nuts grown in Brazil contain selenium?
Do you all tolerate selenium supplementation well? Each time I tried to supplement selenium, I would feel terrible and develop a swelling at the base of my skull, right behind my neck. Maybe a lymph node? This happens, even at very small doses.
Not quite sure what to make of all this. I was diagnosed with Hashimoto’s about the same time I was diagnosed with CFS in 1993. Dr. Atkins, of the Atkins Diet, was the doctor. He put me on T3, saying the problem with Hashimoto’s and T4 was that patients couldn’t convert T4 into T3. So that is not a new finding. They actually tried pure T3 with me first, but it was an enormously complicated regime (involved taking it several times a day). So I ended up on desiccated thyroid, which has done me absolutely no good at all.
Recently a new endocrinologist reduced the amount of desiccated thyroid I was on (which is a combo of T3 and T4, as Cort reports), and added levothyroxine capsules. My thyroid blood levels became perfectly balanced, but I didn’t feel any better.
By the way, there seems to be a big difference in absorption of T4 between levothyroxine capsules (brand name Tirosint, quite expensive and rarely covered by insurance) and levothyroxine tablets (brand name Synthroid, which never did me any good).
I don’t know whether the researchers looked into the difference between those two forms of levothyroxine. I can’t afford the brand name capsules, but the generic capsules (still extremely expensive compared to the tablets) at least makes a difference in the amount of T4 in my blood, so I buy them.
I would be interested in what Cort has to offer on this. Perhaps it was covered in the article, and I just missed it.
Thanks very much for this. The whole subject is important but perplexing.
PS It is possible that the thyroid supplementation is what keeps me too hot all the time. I have thought of stopping it altogether, since it hasn’t made me feel better anyway.
Does anyone else here with ME/CFS not tolerate thyroid hormone supplementation at all (all forms of it, even compounded without fillers)? I get severe hyperthyroid symptoms and psychiatric symptoms even with tiny doses of thyroid hormone, despite my labs showing hypothyroid and hashimotos.
This is probably an adrenaline reaction. This indicates adrenal issues or that nutrient factors are missing. The body can’t speed up the production of energy because the needed cofactors just aren’t there. And that stimulates a cortisol release and adrenaline.
Re-balancing imbalanced hormones can be very difficult, as they all affect each other, and many other biochemicals affect them. Most Endocrinologists have been trained to assume that if TSH, T4 and T3 are in normal range, then there are no thyroid issues. However, Reverse T3 (RT3) is a mirror image of T3 (so it can block T3 cell receptors) so T3 cannot get into cells. When this happens, the TSH, T4 and T3 blood levels can be normal, but the patient can still be hypothyroid (with many symptoms). Unfortunately, too many Endocrinologists treat test results rather than treating patients based on how the patient feels, until their symptoms are resolved.
It is interesting that many other diseases (and biochemical imbalances – notably including iron or adrenal deficiencies) can convert T4 into RT3 instead of T3. In fact, this conversion to (inert) RT3 instead of (active) T3 may happen naturally in animals (humans included) when they are sick or injured – as nature’s way to deprive them of energy – so they will rest (and heal).
Personally, years ago, I had low energy, low heart rate, low body temps, and low BP (all suggesting a sluggish metabolism). My TSH, T4 and T3 were all in normal range. Upon my insistence, my Doc gave me trials of; 1) Natural DessicatedThyroid (NDT), and 2) T4, and 3) T3. Each trial was titrated slowly until reaching very high levels (which only made me “tired but wired”). The trials did nothing for my low energy or for any of my slow metabolism markers. Then I had my RT3 tested (which my doc would not even bother to test). I had high RT3 – which explained all of my symptoms and failed trials – but I could not find an Endo who would treat it or investigate further to find out why my RT3 was elevated. They all just wanted to practice “assembly-line medicine” – giving every potential thyroid patient the same standard cursory tests (starting with TSH. Some will not even test T4 or T3 if TSH is in normal range. Many – like mine – won’t even test RT3 levels). If a patient insists on a Thyroid Supplement trial but does not improve (or improve much) on the standard cursory (usually crude and imbalanced) supplements that they offer – the Endo’s just give up. “Sorry … Can’t help you … Next Patient Please”.
One more observation: Past research (which I have read here) has found that people with CFS often do not have the normal metabolic/biochemical changes that occur naturally in response to exercise in healthy controls. I am wondering if an inadequate adrenal and thyroid response may be amongst the reactions that we should have to exercise, but don’t? (… which may also play a role in our post exercise symptoms?).
I am convinced that CFS and Fibro imbalances many systems, including our hormones. That being said – good luck in finding a Doc who will take the time and effort to re-balance imbalanced hormones in complex cases (which often takes many trials and fine adjustments).
Thx again Cort – for the great reporting!
The answer to lowering RT3 levels is to take only T3. It’s the unused T4 that turns to RT3. There are many reasons why the body doesn’t use the T4 but converts it to RT3 including illnesses unrelated to thyroid problems.
Taking T3 only works well for many people & clears the log jam of RT3. Did you try this approach? It’s viewed with horror by many endocrinologists but that’s based on out dated information & confusion over the very low TSH readings this route causes. Hyperthyroidism causes damage to the body & low TSH readings but the damage is caused by extremely high thyroid hormone levels not by a low TSH reading which is symptomatic not in itself a cause of damage.
You can find lots of information on this by googling Paul Robinson. He has several books out and runs a Facebook page about T3 monotherapy.
TY for your response and information Sue. In answer to your question – Yes, I tried T3 alone (and also tried it in conjunction with adrenal (cortisol) supplementation – as I apparently also had adrenal exhaustion with reversed daily adrenal rhythms, with my highest levels late at night (when I am most awake) and my lowest levels in the morning (when I awaken unrefreshed, and feel like death warmed over)). I tried T3 (only) for a few weeks longer than the best sources (that I could find at the time) said would be necessary to deplete the pool of accumulated RT3. However, I never got past feeling “tired but wired”.
So I gave up on hormone investigations and therapy since all that were available to me had been exhausted – to no avail. At the time I presumed that the problem must be deeper, such as mitochondria that don’t (or can’t) respond – even in the presence of high levels of T3. I was not satisfied that the hormone tests or treatment trials that I had access to ruled out thyroid (or other hormone) imbalances. In fact, numerous tests confirmed several imbalances. However, when none of the treatment protocols available to me helped me (and most just made me feel worse), what choice did/do I have but to look for other (treatable) causes of my symptoms?
Hi, it’s very difficult to find a way through especially since so many doctors etc can have a set viewpoint & don’t seem open to other ideas. You’d be welcome to post your test results on the forum of Thyroid Uk. Many people do, from all over the world & you may receive some useful comments. There is a breadth of experience there which can be extremely helpful.
Cortisol can certainly complicate thyroid treatments. Many say it has to be sorted out first but some, eg Paul Robinson, say taking T3 at the correct time will sort out Cortisol. I’m still struggling having tried many treatments. My latest theory is trying to pinpoint a possible autoimmune component to my difficulties.
This is interesting! I have taken Levaxin for 38 yrs and Lio a few yrs less and I’ve had all the symtoms above all the time (+a few more) including fibro and ME! 4 yrs ago I finished that therapy and started AT (Armor Thyroid) and all my symptoms disappeared successively during the first year! In my opinion, my body does not respond to synthetic hormones, but to natural hormons! I also think, I’ m not the only one with this reaction! Far too many people has the same reaction and gets the wrong treatment!
Hello Pia. I haven’t researched hormones for over 15 years, but when I did, the more reliable sources and Thyroid blogs at the time reported that many (if not most) thyroid patients did better on NDT (Natural Dessicated Thyroid) – such as Armour (which contained T4, T3, T2, T1 and T0, as well as numerous other biochemicals – some of which science may not have even identified yet). In contrast, synthetic hormones usually have only one synthetic (unnatural) hormone. However, in spite of the experience of many patients (and patient blogs) that consistently found NDT to be superior for many patients, Endocrinologists would (generally) just not listen, as they had been trained (or indoctrinated) to prescribe only synthetic hormone replacements. It may be harder to produce standardized dosages of hormones from dessicated natural/animal thyroid sources, but patients didn’t care when it worked better for them.
I was diagnosed in 1993 with Hashimoto’s and CFS by Dr. Robert Atkins, mainly known for the Atkins Diet, but an excellent classically trained MD who had incorporated complementary medicine into his clinic. He told me that Synthroid wouldn’t work for me because patients with Hashimoto’s can’t convert T4 into T3. So he put me on a regimen of straight T3, but I couldn’t follow it because I was still working, at a very demanding job, at the time and couldn’t keep up with the frequent doses. (He put me on selenium, among other supplements, at the same time).
So he switched me to dessicated thyroid (Armour) as the next best thing. I never felt any better, but figured I should take it so I wouldn’t get goiter.
All these years later, a new endocrinologist put me on levothyroxine capsules (important difference in absorption between the expensive capsules and the cheap tablets) in addition to NP Thyroid, which he reduced my longstanding dose of. I don’t feel any better. Perhaps feel worse, but can’t attribute it just to the thyroid supplementation. BTW, the endocrinologist felt his job was complete because my thyroid blood tests all came back picture perfect, (So much for treating to the blood tests rather than to the patient?)
I find the above discussion fascinating, especially re reverse T3 which I had never heard of before. I’ve gone back to my old endocrinologist for Medicare reasons, and hope I can get the RT3 test ordered by him.
One of my consistent problems (which makes it really very hard to live in the modern world!) is extreme heat intolerance. I’ll wilt like a flower without water if the AC isn’t on. The comments above are making me wonder whether to just go off all thyroid supplementation for awhile and see what happens.
I also find the mention of Adrenal Support extremely interesting.
How can we get these tests to be available on the US?
I have had “textbook” CFS and hypothyroidism symptoms for the past 10 years but my thyroid lab results always come back in normal range. Years ago I started to take a bovine sourced thyroid supplement but stopped due to risk of mad cow ds from taking bovine glandulars so I don’t know if it helped or not. Do you think Selenium supplementation would help if thyroid levels are WNL? There are 200mcg of Selenium, as Sodium Selenate, in my multivitamin I take every day but I’m not sure how well it gets absorbed. If it does get absorbed well I haven’t noticed any difference from taking it. The only meds that help me function are steroids. The the topic of auto antibodies is what caught my attention in this article. I really hope this will help those whose thyroid results are out of range as living and functioning with this “invisible” illness is the most challenging part of my life and if this could help any CFS sufferers I would be so happy for them.
I wouldn’t take more selenium until you check out other things first. If I were you my next step would be to find another DR (likely an ND) who can help you take a 24 hour cortisol saliva test (https://www.diagnostechs.com/contact-us/) and see if that’s in range. They can also start you on thyroid meds to see if that helps. I had to change Drs to get thyroid meds when my results were “normal” but they weren’t normal for me. That helped for 15 years before other issues complicated matters. Good luck!
Thank you, yes I like how you said the lab results were “not normal for me” I will have to find a ND in Michigan who is well versed in CFS/ME.
All I can say is ,if you’ve ever had the pleasure of being put through the Canadian medical system,you’ll learn the last thing they want to do is look in depth at anyone’s thyroid.
It’s always …”your t4 and t3 are fine”
“No further testing required”
A 40 year veteran of “the broken system”
“Medical mafia”
I’ve learned the best thing to do is educate yourself,doctor yourself and stay the hell away from them.
Up here ,if they stray off the regimental
system they get big trouble. Of coarse they’ve all got bills to pay (mortgage etc.) So they won’t be off coarse.
I wouldn’t give too much thought on this, as the study does not even mention where the diagnosis of the ME/CFS cohort came from (no mention of criteria at all). Also, the reference studies they use to correlate the two are not good. They correlate the symptomatology of the two diseases as “mental and physical fatigue, poor sleep, depression, and anxiety”. So, they start on a wrong basis.
Explaining the exertional intolerance and PEM ME/CFS patients experience through a lack in thyroid hormone production means they don’t understand what the ME/CFS symptomatology is. Even if they say that their theory would be applicable to only 10-20% of ME/CFS patients.
Sure patients and doctors who may not know how ME/CFS feels, could think that like their thyroid related exertional intolerance and other multi-symtomatology is the same (this may be what Jarret Young has noticed). But there are numerous ME/CFS patients with thyroid issues, so we know that these treatments do not resolve or improve actual ME/CFS, even if they can relieve the extra burden of the thyroid related fatigue.
Lastly, it is a study coming from Charite Berlin and Dr Scheibenbogen -who is an expert in ME/CFS and is from the same institute, has not even posted or commented about it. So maybe she didn’t care about it either.
It is probably just a promotional study for this test (this Dr Schomburg seems to apply the Selenium Ab antibodies to all possible diseases -heart failure, breast cancer etc).
Explaining the exertional intolerance and PEM ME/CFS patients experience through a lack in thyroid hormone production means they don’t understand what the ME/CFS symptomatology is? Since thyroid hormones regulate the metabolism of our cells -= why would you think they might not help to explain PEM?
“They correlate the symptomatology of the two diseases as “mental and physical fatigue, poor sleep, depression, and anxiety” – these are all found in spades in ME/CFS and the researchers discuss PEM later. I’m sorry, this issue is small potatoes IMO.
as the study does not even mention where the diagnosis of the ME/CFS cohort came from (no mention of criteria at all)– If I remmember corrctly the cohort came from doctors office – which is probably better, I would think, than finding patients other ways.
“But there are numerous ME/CFS patients with thyroid issues, so we know that these treatments do not resolve or improve actual ME/CFS, even if they can relieve the extra burden of the thyroid related fatigue.”– what the researchers are suggesting is that 10-20% of patients may not be getting the full benefit. Even if further treatment doesn’t make them well (and I assume more would be needed) – why not appreciate that some benefit might accrue?
Thank you, I appreciate your feedback, as is everyone else here, I am so desperately seeking a treatment that will allow me to achieve a higher level of functioning. Fighting the battle of “moving through quicksand” is a daily struggle.
“Since thyroid hormones regulate the metabolism of our cells -= why would you think they might not help to explain PEM?”
Because Hashimoto patients may experience exertional intolerance and other similar to ME/CFS symptoms, but do not experience PEM. Neither do the breast cancer or the heart failure patients that also seem to express these antibodies in their studies.
This is an extract from a Hashimoto paper about the hypotheses on what these antibodies may do:
“In the absence of regular and efficient SELENOP supply and uptake, severe neurological symptoms, including epileptic seizures, were observed in transgenic mice [ 41, 43–46 ], and also recently in a dog model of impaired SELENOP expression, leading to brain atrophy and cerebellar ataxia [ 47 ].
It remains to be tested whether SELENOP-aAb are relevant for neurological disease, and whether SELENOP-aAb-positive thyroid patients are at particular risk for neurological sequelae, e.g., seizures, tremors, ataxia, or symptoms of Hashimoto encephalopathy.”
It seems that they still don’t know what these antibodies may do and ME/CFS is a good ground to make hypotheses as we still don’t know what drives the disease. However, once more, the above presentations do not paint the picture of ME/CFS. ME/CFS do not end up to these states.
“If I remmember corrctly the cohort came from doctors office”
It is true that the cohort was diagnosed with ME/CFS, but still the study didn’t manage to conclude to a clear correlation to the *subset* of patients who expressed these antibodies. It could as well be coincidental (many patients present with different autoantibodies at times, but the actual correlation is extremely difficult to pin (see the much more relevant and studied GPCR autoantibodies).
Healthy cohorts seem to also express these SELENOP antibodies.
You can’t conclude from the fact that Hashimoto’s patients experience exercise intolerance but do not experience PEM (as anyone assessed that? – I would bet not) that thyroid problems might not cause in another disease. This kind of thing – where the same issue occurs in a different context – and produces different outcomes.
Hashimoto’s is not ME/CFS if it was ME/CFS would be Hashimoto’s.
“what the researchers are suggesting is that 10-20% of patients may not be getting the full benefit. Even if further treatment doesn’t make them well (and I assume more would be needed) – why not appreciate that some benefit might accrue?”–
From the study:
“The samples from controls (n = 119) and CSF patients (n = 111) recruited in Groningen, The Netherlands, had been intensively characterized with respect to biomarkers of inflammation and oxidative stress in a prior analysis [11]. Within this cohort, 12 CFS patients displayed strongly positive SELENOP-aAb (BI > 5).”
We are talking about 12 out of 111 patients with positive aAbs in the NDL cohort, so barely relevant. However, a much bigger group had Se deficiency (not correlated to these aAbs though) so it may be worth supplementing. Other studies have also shown some Se deficiency.
(It may be worth mentioning that in Groningen, The Netherlands where this cohort is from, is where Dr J. Rosmalen -the somatoform advocate- also works and they are infamous for the arbitrary questionnaires they use for ME/CFS diagnosis. Not sure if it is the case here).
Ten percent is not barely relevant – not to that 10%. Plus in the much larger cohort that figure rose to almost 16%. That wasn’t the strength of the study, though. THe strength of the study was the validating work which showed that these patients, in particular, had evidence of thyroid issues, as well as the oxidative stress and iodine findings. In the end it all fit together – that was what impressed me about the study.
Thank you, and yes, if all it took was thyroid medication to treat CFS/ME, we wouldn’t be reading these blogs desperately grabbing at anything that will give us quality of life back. Thank you, as a single mom I am researching and trying to educate myself as much as possible so I can to keep myself functional without going bankrupt. All the multiple supplements I take are expensive and even though I try pacing, working full time and being a mom is unfortunately very difficult.
I don’t think anyone is saying that thyroid medication opr really any one thing is the answer. My guess is that it will often take multiple interventions done at the same time or in the right order to turn things around. The question is whether untreated thyroid problems that may be exacerbating ME/CFS – causing more fatigue, more exercise intolerance, etc. – might be present. The thyroid situation is complex; this isn’t the first time that thyroid issues that most doctors aren’t aware of have been raised in ME/CFS/FM.
Yes, it’s pretty clear to me that my hypothyroidism (pre ME/CFS) wasn’t being treated well enough on thyroxine/T3, and after some years on it, many of the classic signs of the original issue had returned – constipation, severe fatigue, deepened voice, low body temperature had returned. As a result I simply gathered a new diagnosis of ME/CFS.
Going onto T3 monotherapy has resolved all of the hypothyroid symptoms (some 15 years after my original diagnosis of the thyroid issues). It has improved many of the ME/CFS symptoms (along with other things such as natural progesterone, supplements, good diet, sunshine), but I still have PEM if I exert myself and have to rest afterwards. I also have had additional symptoms recently which I think were post viral – so on top of the ME/CFS issues that are always there. Thankfully, with Spring here, I am beginning to improve again.
I am however fortunate that I am now older, and am retired, as having the complications of work, inability to pay for things to aid health, and bringing up children, can be really detrimental to healing. I had my worst of ME/CFS just after menopause, but was fortunate to not develop it while my children were young. I was also lucky to at least get diagnosed with an underactive thyroid as that can be another battleground, and the first GP I went to with symptoms refused to even test me.
Thanks for relaying your story and congratulations on the thyroid win 🙂
Maureen, I’m so sorry that you are struggling! And you are right that thyroid medication alone will not “cure” CFS/ME. But until the underlying cause is found, all we can do is treat symptoms. What is helpful about this study is that it identifies one of those “hidden” symptoms (low T3) that contribute to making you feel so crappy. I have FM, and taking T3 definitely didn’t solve everything for me, but it was a big help. It doesn’t hurt to ask your doctor to test your TSH/T4/T3 (all three) and see if you have low T3, for which liothyronine (cytomel) could help you, too!
Wondering if this could e.g. result in a signal in GWAS [common genetic variants – which have a low to modest effects – DecodeME] and/or rare variant genetic study (whole genome sequences – higher impact variants)? Check out the NIH genetics webinar – one of the suggested research priorities was a rare variant [whole genome sequence] genetic study e.g. families with more than 1 member affected
Yet another antibody found in a CFS population. Just like with adrenaline and acetylcholine receptors. Just like the body’s response to nutrient intolerance. It seems that the immune system attacks a certain continuous flow of substances. This is a consequence of the disease ME/CFS/POTS, not a cause. B cells do not work properly ?
Dear Cort, I’m quite delighted to finally have an explanation why I need the thyroid regime the late Dr Peatfield helped me to set in place.
I’m on 100 – 115 mcr liothyronine plus one natural thyroid capsule. I have to adjust the t3 according to time of year. T3 should be at its highest somewhere around 5 in the morning and I set my alarm for 3, take 50 – 65 mcg and sleep on. When I wake in the morning I take another 50, 3 in the afternoon about 10 – 15. At bedtime I take the natural thyroid capsule. And I do take selenium.
I’ve been on this since 2007, and it suits. In 2006 I was diagnosed (after 50 years Hashimoto’s) and started on combination T4/T3. Eventually I ended up both hypo and hyper at the same time .
TSH is not very relevant as there is no feedback to the pituitary from peripheral tissues. The cells simply take from circulation what they need , if they’re able to. Population studies tell us that healthy people have a TSH of 1, reference interval is invalid as it’s not a bell curve.
Dr Peatfield told me he went to the Broda Barney’s foundation in the US to train, then learned from the Hertoghe family of endos in Belgium. They have been in it and on top of it for 4 generations.
It has been established that thyroid medication does not cause palpitations. But ME might cause vagal palpitations, or mast cell activation can cause it too. We’re vulnerable to those.
I’m sure there’s more to know, and now I hope to put my gp’s mind to rest with this.
I have fibromyalgia. I saw a rheumatologist first, but she only tested my TSH, which was normal. (She also lied to me, which she admitted was out of laziness, so I “fired” her, but that’s another story). Fortunately, I found a new doctor (primary care, functional medicine) who did the full spectrum of thyroid testing. She found that although my TSH and T4 were indeed normal, my T3 was very low. She started me on a T3-only prescription, which had to be carefully monitored until we found the right dose, but I feel so much better!
Thanks, this work is excellent! I had already read the research paper, but with your well-written summary, I learned more about the workings of thyroid at the cell level. I already shared this with my sister who also has CFS.
Thanks! Stay tuned for part II 🙂
This is really helpful information! I was diagnosed with Hashimoto’s about 7 years after my Addison’s diagnosis – TWO autoimmune diseases! My doctor put me on Armour thyroid and I stayed on that for many years. But a year after the Hashimoto’s diagnosis, I got a severe CMV infection that triggered my worst CFS. At some point (I really don’t remember when), the Armour became unavailable, so my doctor switched me to levothyroxine, and I felt horrible. After dragging around for several months, he added liothyronine, which helped a lot. To be sure, it didn’t cure the CFS, but I was able to function better. This article indicates that there may be yet another autoimmune problem – with the T4-T3 conversion. Good to know! But it’s not clear whether selenium supplements might help here – any info available on that?
Hi Cort: I read this article sometime ago, and as a longtime thyroid patient, currently on T4/T3 therapy (porcine thyroid, brand name “AdThyza”), I concur that something is happening with both deiodination, and perhaps T3 resistance (antibodies to triiodothyronine perhaps?) as well in LongC. Note also that the blunted/altered cortisol response in LongC may be T3 dependent as well. I have observed that I feel my absolute worst in the a.m. Roughly three hours after my a.m. meds, I will feel somewhat functional most days. However, I’ve also observed, if I try to raise meds beyond the dose my body currently tolerates, I can get anxiety/adrenaline dumping in the late afternoon.
If you haven’t read Antonio Bianco’s book, “Rethinking Hypothyroidism” or listened to his NPR/”People’s Pharmacy” interview with Joe and Terry Graedon, both are worthwhile. I wonder if the genetic differences in people that are “deiodination challenged” may be a factor in LongC/LongV/CFS/ME. Attaching the NPR interview here: https://www.peoplespharmacy.com/articles/bonus-interview-dr-antonio-bianco-describes-his-remarkable-thyroid-research
Also, there is a Part 2 blog coming on this subject, correct? I look forward to reading, and as always, am grateful for all you do.
Thanks for the link to the interview and book! There is a part 2 coming up 🙂
We are eagerly waiting, do you know when part 2 is ready?
Thank you, thank you, thank you!
Someone posted a link to your post on facebook, and after reading it several times I ordered selenium to try this out.
I’m already on NDT and liothyronin and have now been taking selenium for about 2 months. The difference in my wellbeing is enormous! This has made a huuge difference for me, and I can’t thank you enough!
I’ve been in hell for 7+ years and this has given me hope that I one day can be my normal self again.
Thank you!