

Geoff’s Narrations
The Blog
The GIST
The 2025 3rd International ME/CFS and Long COVID conference, put on by the Charité Fatigue Center at Charité —Universitätsmedizin and supported by the ME/CFS Research Foundation from May 12-13, 2025, was impressive indeed. As fascinating as many of the findings were, equally impressive was the fact that most of them came not from the US or the UK but from Europe, which, not too long ago, was not exactly leading the pack in ME/CFS research. The rise of Europe as a significant center for ME/CFS and long-COVID research is encouraging, as is the involvement of many younger researchers.
THE GIST
- The interesting (and perhaps fundamental?) findings from the 2025 3rd International ME/CFS and Long COVID conference highlighted the emergence of Europe as a significant center of ME/CFS and long COVID research. It was encouraging to see the number of younger researchers involved.
- The Cardiovascular Dysregulation and Mitochondrial Pathology” section was led off by David Systrom who reviewed his major findings: reduced preload (filling of the left heart) in just about everyone and problems with oxygen extraction in a significant subset.
- After doing a deeper dive in the small nerve fiber problems in ME/CFS Systrom (paper coming up) Systrom found a significantly higher prevalence (60%) than previously reported. He suggested that the SFN may be playing a role in preventing blood (oxygen and nutrients) from reaching the tissues.
- The increased “collagen deposition” finding from a young Anouk Slaghekke at Rob Wust’s lab may have been the hit of the conference. Stating she’d never seen anything like this before, Slaghekke found high levels of collagen deposits in the smallest blood vessels (capillaries) and other structural problems (massive basement membranes, capillaries with blocked or narrowed passageways, vacuoles).
- All of these could impair the precious flow of oxygen and nutrients into the muscles and help explain the problems with energy production. Saying he was “super-excited” by the findings Rob Wust noted that they showed the most clear-cut difference seen yet in his lab between healthy controls and the ME/CFS patients.
- In an update of his and Fluge and Mella’s model of several years ago Dr. Karl Tronstad reported that increased lactate levels (a sign on anaerobic energy production) had been observed at a very low workload (2019 study). The utilization of unconventional energy sources (e.g., amino acids) suggested that hypoxia (low oxygen levels) were causing a shift from aerobic to anaerobic energy production.
- Energy-sensing pathways, such as mTOR, AMPK, and SIRTs, then instruct the cells to reduce oxygen consumption, and glycolysis (anaerobic energy production) ramps up, producing lactate that contributes to exercise intolerance.
- They believe three metabotypes exist in ME/CFS. One mimics what happens in starvation or fasting, impaired carbohydrate metabolism, or mitochondrial dysfunction, and involves the body shifting from relying on carbohydrates for energy to relying on fats and amino acids.
- The second one is associated with impaired utilization of fatty acids by the mitochondria. Instead of being used up, lipids are stored in the tissues, leading to insulin resistance, particularly at the tissue level. Fat deposition in the liver, muscles, and other tissues produces inflammation and cellular stress. At the tissue level, a state of “pseudostarvation” occurs. This group is the worst off. The last group is a mixture of the first two.
- Coming soon from this group, expect a proteomic analysis of ME/CFS patients’ serum, which may help explain why simply adding their serum to cultures causes so many abnormalities.
- Christian Puta proposed that immune activation including deformed red blood cells were driving the microvascular (capillary) problems in ME/CFS. These microvascular problems were then preventing blood and oxygen from reaching the mitochondria in sufficient amounts.
- Puta became aware of the deformed red blood cells in COVID-19 when a colleague told him he’d never seen anything like this. Not only are the deformed red blood cells stiffer, which prevents them from entering the probably already quite narrowed capillaries, but they appear to be hanging on tightly to the oxygen they’re carrying.
- In conclusion, it appears that multiple barriers to blood flows and the diffusion of oxygen/nutrients to the tissues (not to mention the removal of waste) are present in these diseases. They include thickened capillaries and muscle fibers, reduced capillary density, endothelial cells with narrowed or even blocked passages for blood flow, deformed and stiffened red blood cells, and blood cells that are tightly clinging to their oxygen molecules.
- All could make normal levels of energy production impossible. Multiple barriers would make perfect sense in diseases that can produce such debility.
- The reliance on non-anaerobic means of producing energy that results is reflected in Wust’s finding of increased glycolytic (non-aerobic) fibers at the muscle level, Tronstad’s glycolytic model, and Puta’s glycolytic findin.gs
- While the conference did not mention it, it’s also possible that increased collagen deposition in capillaries could be impairing oxygen and nutrient diffusion to tissues (including the brain) throughout the body, and that increased collagen deposition may be occurring in other tissues.
Please Support Health Rising and Keep the Information Flowing
HEALTH RISING IS NOT A 501 (c) 3 NON-PROFIT
Understanding I: Cardiovascular dysregulation and mitochondrial pathology
I always thought the muscles were going to be a big part of these diseases…but that’s getting ahead of things. First, a top-down look from David Systrom.
David Systrom – “Circulatory Dysfunction in ME/CFS”
David Systrom’s presentation was an excellent choice to start off this section because it provided a more systemic overview of what’s happening in these diseases. We’ll see that later presentations may be able to explain, on a more microscopic level, why Systrom has found what he has.
Systrom’s invasive exercise test results have redefined what we know about these diseases. Because the invasive exercise test examines what happens to the blood as it makes it way from the heart to the muscles and then back to the heart, it provides an unparalleled way to understand the energetic system in ME/CFS and long COVID, in broad strokes.
Systrom came to ME/CFS independently of the field when he began examining people with unexplained exercise intolerance. He first showed up on the ME/CFS scene with a poster at the 2014 IACFS/ME International Conference. His first paper on the subject, “Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing“, contained data from over 600 people with exercise intolerance and did not mention ME/CFS (apparently because he did not have diagnostic data on all of them).
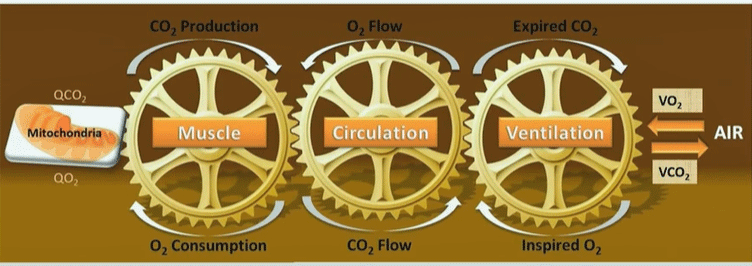
Systrom’s macro model of energy production.
The next data set in 2021 was even larger (@ 1,500 people) and did focus on ME/CFS. He identified several subsets: in one subset, impaired blood flows caused by preload failure were the major contributor to their depressed energy output during exercise.
Preload Failure – THE Ubiquitous Finding in ME/CFS and Long COVID
Systrom noted that the one ubiquitous finding in ME/CFS and long COVID is “preload failure”. Preload failure refers to reduced “filling pressure” when patients are upright, and particularly during maximum exercise. Preload failure occurs when the veins that return the used-up blood to the heart fail to constrict properly and drive normal amounts of blood to the heart.
The preload failure sets up reduced stroke volume, which refers to reduced amounts of oxygenated blood flowing out of the right side of the heart into the arteries.
Preload Failure + Impaired Oxygen Uptake
The other major subset has preload failure plus an inability to take up oxygen in the muscles. This group is not pumping enough blood out, and the oxygen in the blood they are pumping out is not being taken up – a double whammy – as Systrom put it. (An upcoming paper from Systrom will show that the same thing is happening in long COVID.)
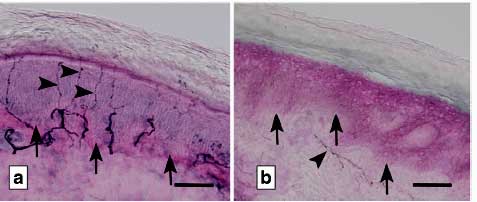
Systrom’s more extensive study of small fiber neuropathy (SFN) bumped the incidence to about 60%. SFN could be impairing blood flows to the tissues.
The big question, of course, is what is happening here? Possibly, enter small fiber neuropathy (SFN) which refers to damage to the small unmyelinated nerve fibers that transmit sensory and autonomic nervous system signals. Systrom has a new, and yes, large (n=407; Systrom seems to specialize in large studies) SFN study coming out.
There’s no shortage of SFN studies, particularly in fibromyalgia, but this isn’t your normal SFN study. Systrom went deeper to access the sweat glands as well, and when he did, so he found the highest prevalence yet of SFN – a whopping 50% increase from 40 to 60% prevalence- in these diseases.
Systrom noted that he initially thought that a peripheral “left-to-right shunt” was to blame. A left-to-right shunt occurs when oxygenated blood from the left side of the heart flows back into the right side of the heart, thus bypassing the lungs. That would explain how the high oxygen levels he’s found are leaving the heart and then entering the heart.
Some left-to-right shunting does appear to be occurring, but a host of other explanations have shown up over time, including a small fiber neuropathy that shunts blood away from the muscles, capillary problems – now a major possibility (see below), and mitochondrial issues that prevent them from taking up oxygen.
Anouk Slaghekke’s “Skeletal Muscle in ME/CFS and long COVID” Poster and Presentation
“I’ve never seen collagen deposition like this before.” Slaghekke
I was on the edge of my seat with Anouk Slaghekke’s “Skeletal Muscle in ME/CFS and long COVID” presentation.
Slaghekke’s poster, “Microvascular Dysfunction and Basal Membrane Thickening in Skeletal Muscle in ME/CFS and Post-COVID: from Pathology to Diagnosis“, may have been the hit of the conference and was one of three to win an award at the conference.
Wust said Slaghekke had called him over, saying, “I’ve never seen collagen deposition like this before”. Wust said he was “super-excited” by her findings because they showed the most clear-cut difference seen yet in his lab between healthy controls and the ME/CFS patients.
Slaghekke pointed out that the amount of oxygen and nutrients reaching the muscles is not just a matter of blood flow, but that something called diffusion is equally important. Once the blood gets to the muscles, oxygen and nutrients need to be able to diffuse across the capillaries into the tissue itself.
Using an electron microscope, Slaghekke assessed muscle biopsies for capillary levels, collagen IV content, and structure. First she found “increased collagen IV deposition in the capillary basement membranes”.
Four types of collagen exist. Collagen IV provides the main structural support for the “basement membranes” – connective tissues that underlie the blood vessels, muscle fibers, and epithelial (skin) cells. Too much collagen deposition in the capillaries would make them rigid and impair their ability to provide blood, oxygen, and nutrients to the muscles (and remove waste products from them).
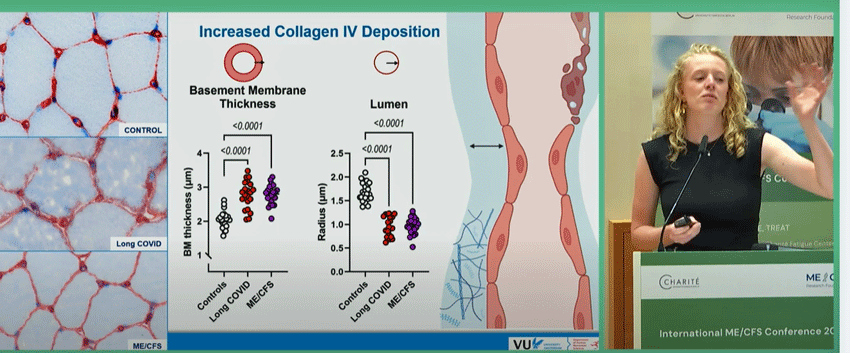
Note the clear capillaries in the healthy controls vs the collagen-loaded and cloudy capillaries in the ME/CFS and long-COVID patients. Then check out how well differentiated the ME/CFS/long-COVID patients were from the healthy controls. Finally, see the narrowing of the lumen – the passage through which blood flows – in the ME/CFS/long-COVID patients. (The basement membrane is in blue).
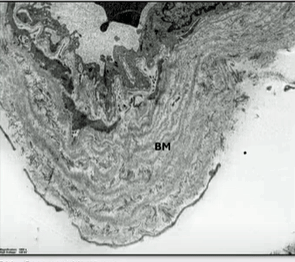
Check out the massive basement membrane (BM) found in an ME/CFS/long-COVID patient.
Digging deeper, Slaghekke found, using electron microscopy, at times massive basement membrane thickening, vacuoles, and endothelial cells that were so thickened that no lumen (no interior) was present through which the blood could move (!).
All that spelled problems diffusing the oxygen and nutrients out of the capillaries into the muscles and removing waste products from the muscles.
While Slaghekke’s finding was in the muscle capillaries, one wonders if increased collagen deposition is occurring in other capillaries or other tissues. Increased collagen deposition in the blood vessels could be impairing blood flows in the brain, disrupting the blood-brain barrier, and allowing waste products such as amyloid proteins from being cleared. Increased collagen deposition has been found in liver, kidney, and lung disease, scleroderma, vascular diseases, and aging.
A 2023 German study found a reduction in the number of capillaries feeding the muscles, increased expression of extracellular matrix (connective tissue) genes, thickened cell basement membranes, as well as in what the authors described as a “remarkable” number of inflammatory macrophages (CD169+) and complement proteins. The authors proposed that extracellular matrix problems were also likely to be found in the muscle fibers.
These findings align well with Wust and Slaghekke’s observations of reduced capillary density, damaged capillaries, and evidence of immune infiltration, which may contribute to the damage.
Amyloids (strangely shaped, difficult-to-break-down proteins) provided an interesting side note as they could reflect and inability to remove unwanted materials. Wust noted they were investigating an amyloid product they’d found in the muscles, and amyloid proteins have shown up in ME/CFS studies before.
Wust noted they are looking for collaborators to validate their findings.
Jürgen Steinmacker – Changes in the Mitochondria in the Muscle
Prof. Dr. Jürgen Steinacker (University of Ulm) has been engaged in the EPILOC study that’s been charting the long-term effects of long COVID. EPILOC, which has mostly focused on symptom persistence thus far, has changed gears a bit, and recently delved into a most interesting part of ME/CFS and long-COVID pathophysiology: the mitochondria.
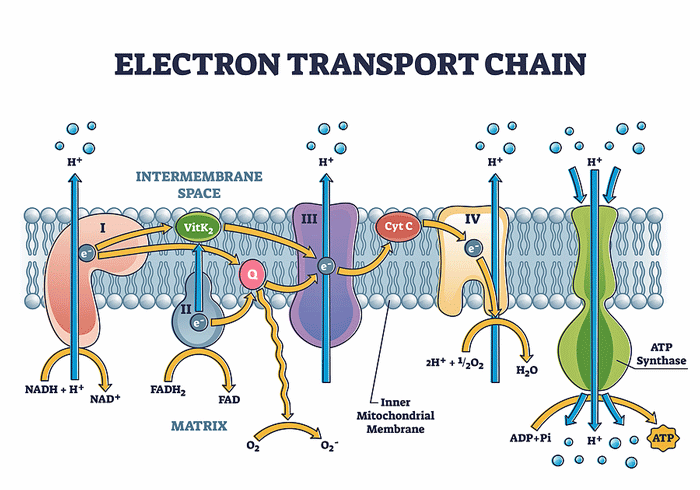
Four (or five, depending on how you are counting) complexes exist in the electron transport chain. Studies have suggested that different complexes are impacted in these diseases.
Steinacher published “Functional and Morphological Differences of Muscle Mitochondria in Chronic Fatigue Syndrome and Post-COVID Syndrome” in 2024. The study found decreased activity of the first complex in the electron transport chain in the mitochondria in both long-COVID and ME/CFS patients, as well as morphological changes in cristae (folds). Steinacher noted that the coronavirus downregulates mitochondrial genes, and, in fact, many viruses, including cytomegalovirus, hepatitis C, and respiratory syncytial virus (RSV) have been found to do that.
Decreased activity of the first complex results in reduced ATP production (energy production) as well as increased oxidative stress. Decreased complex I activity in aging – which may be happening prematurely in these diseases – is associated with ataxia (clumsy movements) and microglial activation.
(Findings concerning the complexes in the electron transport chain have been variable, however. Missailidis found reductions in Complex V activity and increased Complex I activity.)
The lack of a correlation between mitochondrial function and aerobic capacity in the long-COVID group suggested, as did Rob Wust’s recent paper, that mitochondrial problems, while present, may not be the be-all and end-all of the energy production problems in these diseases.
Steinacher, though, proposed that stress reduced levels of heat shock protein 70 (Hsp70) could “immediately” reduce mitochondrial energy production in these diseases. Heat shock proteins protect the cells from oxidative stress, and indeed, in 2009, Jammes, in a small study (n=18), found that ME/CFS patients with low levels of Hsp70 evidenced the highest amount of oxidative stress following exercise.
Karl Johan Tronstad – Changed Patterns of Blood Metabolites
Lost in all the excitement over Rituximab was all the other work that Fluge, Mella, and Tronstadt have been doing elsewhere with ME/CFS. They were early investigators exploring the metabolic basis of the energy production problems in ME/CFS, assessed endothelial functioning, and were one of the first, if not the first, to find that something in the ME/CFS serum caused increased oxygen consumption and triggered lactate production in muscle cells.
Back in 2021, Dr. Tronstad and his Norwegian colleagues published a large study, “A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome“, suggesting that “elevated energy strain” caused by exertion-triggered reductions in tissue oxygen levels (tissue hypoxia) played a core role in ME/CFS. That same year, Tronstad, Fluge, and Mella introduced a working hypothesis in “Pathomechanisms and possible interventions in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)”
Their pathomechanism paper proposed that three main factors were present:
- An infection triggered an immune response, and featuring the B-cells, was present
- The B-cells produced autoantibodies that damaged the blood vessels and the autonomic nervous system.
- Attempts to compensate for the damage resulted in an increased sympathetic tone (increased fight-or-flight response) and metabolic adaptations that attempted to restore energy production.
Now, four years and a lot of ME/CFS and long-COVID research later, he (and Fluge and Mella) were back with an updated version of their understanding of what’s happening in these diseases.
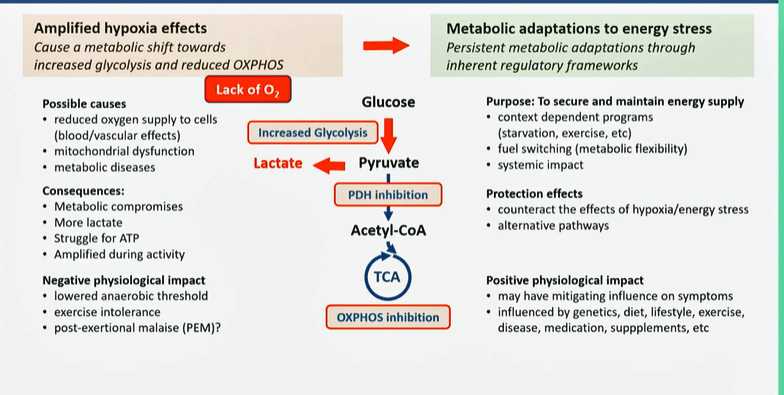
Tronstad’s Pathomechanism model for ME/CFS.
Tronstad quickly skipped over the blood vessel findings – four years later, it seems fairly clear that the circulation is impaired, not just in the muscles but also in the brain.
At the metabolic level, Tronstad focused on the increased lactate levels observed at a very low workload (2019 study) and the utilization of unconventional energy sources (e.g., amino acids). As they proposed four years ago, hypoxia (low oxygen levels) causes a shift from aerobic to anaerobic energy production.
Energy-sensing pathways, such as mTOR, AMPK, and SIRTs, then instruct the cells to reduce oxygen consumption. Glycolysis (anaerobic energy production) ramps up. Instead of pyruvate going to acetyl-CoA (and aerobic energy production), it produces lactate, and ultimately, exercise intolerance. Glucose is conserved, leaving amino and fatty acids to produce energy. In some cases, dyslipidemia and insulin resistance pop up
Variations of this model explain why some patients respond to efforts to improve oxygen levels (such as hyperbaric oxygen treatment, drugs, supplements, or physical exercise) while others don’t.
While they believe that tissue hypoxia is found in everyone with these diseases, three “metabotypes” are found:
- Metabotype 1 – has increased NEFAs and ketone bodies; low triglycerides. This metabotype occurs when the body shifts from using carbohydrates to using fats as its primary energy source. It’s associated with starvation or fasting, prolonged exercise, impaired carbohydrate metabolism, or mitochondrial dysfunction. (On a personal level, this metabotype interested me because of the very low body weight I experienced during my ME/CFS onset, which resulted in gynecomastia (thickening of the breast tissue). While the metabolic changes in this metabotype do not directly produce gynecomastia, they can suppress male hormone production and result in gynecomastia.)
- Metabotype 2 – high triglycerides, low NEFAs – similar to dyslipidemia; in this metabolite, impaired utilization of fatty acids by the mitochondria diverts lipids toward storage. Insulin resistance is present, particularly at the tissue level. Fat deposition in the liver, muscles and other tissues drives inflammation and cellular stress. “Pseudostarvation” occurs at the tissue level. This group is the worst off.
- Metabotype 3 – the smallest subset (@ 15%), metabotype 3 overlaps with 1 & 2; the patients are less affected overall.
In their latest work, soon to be published, a proteomic analysis of ME/CFS patients’ serum uncovered major changes in the immune, secretome, and metabolic proteins. They believe the secretome proteins could be a key finding as they can affect the entire body. All in all, the findings point to immune dysregulation that affects the blood vessels and the metabolism.
Christian Puta – Mechanisms of Post-Exertional Malaise
“I’ve never seen anything like this before.” Wilhelm Bloch
Now we turn to Dr. Christian Puta at the University of Jena for his model of how post-exertional malaise (PEM) is produced.
Earlier this year, Puta and 23 other researchers from Universities across Germany published “Towards an understanding of physical activity-induced post-exertional malaise: Insights into microvascular alterations and immunometabolic interactions in post-COVID condition and myalgic encephalomyelitis/chronic fatigue syndrome“.

Christian Puta started his presentation off with a short stand-to-sit exercise.
Explaining that he will soon get to the exercise test, Christian Puta started his presentation off by leading the audience in 10 “sit to stand” (arms crossed on chest) squats. Afterwards, like Tronstadt, he warned there is “no universally valid explanatory mechanism” but that a number of different factors work together in various ways to produce ME/CFS in different people. As with Systrom, Slaghekke, Wust, Tronstad, Fluge and Mella, he proposed that problems with oxygen – in this case oxygen extraction – plays a central role in these diseases.
Puta started off by suggesting that immune activation, including deformed red blood cells, were driving microvascular (capillary) problems which prevented blood/oxygen from getting to the mitochondria in the proper amounts.
Factors produced during exercise (lactic acid, reactive oxygen species (free radicals), prostaglandins, Na+ K+ ATPase dysregulation, b2 AdR autoantibodies) might be systemically dysregulating the immune system.
He turned to David Systrom’s work showing that peak venous succinate and peak venous lactate were correlated with peak energy production (VO2 max) at about 4 “METS” of activity. (Four METS corresponds to the sit-to-stand exercise.) Because succinate is released into the bloodstream during periods of high metabolic stress and reflects impaired mitochondrial function, the study suggested that ME/CFS patients’ mitochondria would have buckled doing the sit-to-stand exercise.
Deformed Red Blood Cells
He then turned to call a colleague, Wilhelm Bloch, who told him, referring to the deformed red blood cells he was finding in COVID-19, “I’ve never seen anything like this before”.
In 2021, Bloch and colleagues published an incredibly complex paper, “The oxygen dissociation curve of blood in COVID-19“, which, if I have it right, demonstrated an increased affinity of oxygen in hemoglobin in COVID-19. This suggests that oxygen is binding very tightly to hemoglobin (probably caused by increased levels of methemoglobin) – making it more difficult for oxygen to get off the red blood cells and diffuse into the tissues.
If that wasn’t bad enough, Bloch’s 2022 paper suggested that increased oxidative stress and stiff, less deformable red blood cells were also impairing oxygen diffusion into the tissues in people with mild long COVID. (Bloch’s finding was recently validated, particularly in females, in an Italian study which concluded:
“Persistent alterations in RBC functionality may underlie the microvascular dysfunction and symptoms of post-acute COVID-19 syndrome (PACS), including fatigue and cognitive impairment.”
Laughing, Puta thanked a patient who informed him that Leslie Simpson, a New Zealand researcher, found problems with red blood cell deformability in ME/CFS over thirty years ago. Actually, the first mention of red blood cell deformability problems in ME/CFS appears to have occurred in a Letter to the Editor of Lancet, “Abnormal Red-Blood Cell Morphology in Myalgic Encephalomyelitis” in 1987 (!).
Interest in the subject died with Lloyd’s failure, in an achingly small study (n=22), to validate Simpson’s findings. Simpson, however, persisted and after assessing red blood cell morphology in over 2,000 patients, wrote in a 1997 paper: The results reported here “would support a proposal that ME is a hemorrheological disorder in which symptoms are manifestations of the consequences of impaired capillary blood flow.” Twenty-two years later, a 2019 Stanford/San Jose University study found “stiffened” red blood cell membranes in ME/CFS.
Conclusion
What an interesting session this was! It indicated that European researchers are pushing the ME/CFS field when it comes to the muscles, mitochondria, blood flows, diffusion issues, and red blood cells.
While the blood flow findings, from the heart to the capillaries, appear to be mounting, the mitochondrial problems at the heart of these diseases seem to recede somewhat from center stage. Both Wust and Steinmacher found mitochondrial abnormalities but concluded that more was going on.
Note how many blood flow (read oxygen/nutrient flow) problems showed up (reduced preload/stroke volume, reduced number of capillaries, collagen deposition in the capillaries, deformed red blood cells, tightly held oxygen molecules on hemoglobin). While microclots were not mentioned, they provide another potential barrier to blood flows.
Next came numerous possible reasons that could explain this, including thickened cell basement membranes in the capillaries and muscle fibers, endothelial cells with narrowed or even missing passages for blood flow, and reduced capillary density. All of these suggest that barriers to oxygen and nutrient perfusion are impairing energy production in these diseases.
As the body tries to make energy without using oxygen, Wust’s finding of increased glycolytic fibers at the muscle level, Tronstad’s glycolytic model, and Puta’s glycolytic findings make sense.
When you add in deformed and stiffened red blood cells, as well as red blood cells that cling very tightly to their oxygen molecules, it appears that multiple barriers to oxygen and nutrient diffusion may exist in numerous tissues in these diseases. Multiple barriers would make perfect sense in diseases that can produce such debility.
Please Support Health Rising and Keep the Information Flowing
HEALTH RISING IS NOT A 501 (c) 3 NON-PROFIT






‘ I’ve never seen collagen deposition like this’.
Sounds about right
Some nutritional expects emphasize the role and benefits of collagen – to keep healthy people healthy. I have tried supplementing it several times on different occasions over the years, but it has always made my symptoms notably worse. This research offers a potential explanation.
The article also spoke of 3 different metabolic types of patients . I think I am type number 2 (the worst type), but I may have been type number 1 during the first decade or so of my illness with CFS. I am wondering if patients can change from one type to another during the course of the disease? (and perhaps back again?).
I am also wondering if chronically low (or lower) blood pressure might play a role (perhaps as a predisposing or exacerbating factor) in the oxygen deficiency/circulation problems described in this research? I wonder because my blood pressure was always significantly lower than normal, even during my childhood before my symptoms became clinical.
Thank-you for the excellent synopsis Cort!
Supplementing with collagen us ok for nutrition for some people although some people have mast cell reactions to them.
Collagen type is smthg you’re born with. All your connective tissue is made of it as you probably know.
Love blood pressure appears over all cohorts and it’s not a defining factor for cfs patients.
This is smthg deep. It runs in families and it needs to be addressed at that fundamental level.
Of course, like everyone here, I’ll take any treatment that eases our misery.
But I’m certain it’s a phrnotypic dideqse.
We’re more prone to the cell danger response during to our make up. Literally everything else is downstream of that initial phenotype.
Some people who are say prone to heart disease might have genetic alterations that cause high cholesterol.so they may eat a bad diet and get sick, so the predisposition to it was always there.
It’s the same with our collagen phenotypes. It predisposed is to leaky gut, mast cell hyperactivity, autoimmunity, autistic features. But all those predisposition are downstream of how we’re made up at conception
TY for your reply Oliver. I have all of the symptoms that you described as common due to an abnormal collagen phenotype, processing, deposition or function. However, I have never (before) looked into collagen as a potential contributor to my symptoms. TY for the head’s up. All the best to you.
No problem. Look at the rccx theory too that describes how many conditions such as ibs, autism, autoimmunity, etc all share common collagen genes.
Also check out how similar eds can be to m.e. I mean heds, type 3 eds looks virtually identical in expression and often onset. To me it’d a question of the severity of distribution of collagen and collagen type that leads to disease severity for the most part.
Earlier this week I was reading an article about how an array of blood tests revealed distinct differences in the blood of p/w/ME/CFS.
It seems I have read a lot of articles whose findings reveal distinct differences between p/w/MECFS and those without.
So, as a layman, I have struggled to understand why–after all these findings–there is not a diagnostic test (even an expensive one).
The tests you talk of were pretty basic. We kinda looked like someone with diabetes and non fatty liver disease. The other readings were basically like a form of mild poisoning. It wasn’t enough to separate us from people with many diseases but it did prove there I’d smthg going on with is as a cohort
Systrom’s research is definitely amongst the most promising
Bloch’s 2022 paper suggested that increased oxidative stress and stiff, less deformable red blood cells…
I can’t figure out if we are dealing with excessive glycosylation or low levels of glycosylation. Add to the mix that levels of asparagine levels are low in ME/CFS.
impact of glycation on erythrocyte structure
https://pmc.ncbi.nlm.nih.gov/articles/PMC7337333/
Glycosylation, the process of adding sugar molecules to proteins, can reduce the deformability of red blood cells (erythrocytes). This occurs when high glucose concentrations in the blood cause proteins on the erythrocyte membrane and hemoglobin to become glycosylated. The resulting stiffening of the membrane and increased internal viscosity due to glycosylation can hinder the ability of red blood cells to deform and pass through narrow capillaries, potentially leading to microcirculation problems and reduced oxygen delivery.
At the moment I’m experiencing slight low blood oxygen levels, hypoxemia. Only slightly drops to 93, odd time 92, when its stabilised it’s usually 95, so never on the higher side of the range 95 to 100, range here in UK.
When it drops in night, I tend to wake up, dripping wet, so my body is compensating I recon. Now blood oxygen levels dropping usually I believe is breathing, lungs. I have no lung issues or breathing issues. I have episodes of Tachycardia, and recently a night of what felt like many missing heart beats. Ive had echo that came back as normal. Nothing showing on ECG but they never catch anything.
I’m perplex as to what causes the drop in blood oxygen levels. Whether it’s my heart starting to weaken die to lack of movement, I’m severely affected for 13 years, or whether it’s some M.E issue I dont know.
From my little understanding hypoxia is more likely heart issues, oxygen in blood but not getting into muscles, but hypoxemia is getting oxygen in blood in first place and usually lungs and breathing, although I’ve read it can also be heart issues as well, but not sure why or how.
I dont feel lower blood oxygen is causing me any particular issues other than I wake up sweating, but it’s no ideal, and long term it may well be more serious, but at the moment it’s not always dropping, its just in normal range at 95, so I’m trying to figure out what is triggering these episodes of it dropping.
If I take some deep breaths it rises, so I was wondering whether I’ve become accustomed to shallow breathing, especially at night so I am not taking in enough oxygenated air, who knows, this illness is baffling.
Some great stuff in this article, just wish we get some answers and solutions that will finally help us recover or at least improve our lives
That’s interesting about the night sweats when your O2 levels go down. I have had night sweats and never figured out why.
Anemia can cause night sweats. And I just saw an article about iron dysregulation caused by COVID, and Long Covid is very similar to ME/CFS.
So I have been considering iron deficiency for a couple of weeks now, and started taking a non-heme iron supplement. I have had at least one soaking night sweat that I can remember since I started taking it. That happened after taking an alka seltzer, and alka seltzer had caused night sweats for me more than once. (This is just a tangent that I have to think about.)
At least you know that the hypoxia is related to low blood levels. That’s a good start. But I don’t know what solution that would point to, because as you said, deep breathing helps.
I actually havent had a diagnosis. From research i understood low blood oxygen, which is what is happening to me is called hypoxemia, which is different to hypoxia, which is where oxygenated blood doesn’t get to muscles. So on that I assume I have hypoxemia.
I am not sure why, because from my understanding it’s usually to do with breathing, however there has been some research saying it could be heart related, but its seems complicated to me. I no idea what tests you have to have to know if its heart related. I had a echo due to the Tachycardia I get, and that was normal. I’ve had paramedics out and ECG at the time are fine, although the last one I was Tachycardia, but no obvious signs as to why I am Tachycardia. I was supposed to have a herat monitor fitted but seems to have got lost in the system as it’s been over a year waiting.
I havent pursued it because the trip to the hospital for the echo over eighteen months ago, I havent recovered, I just wouldnt cope with another trip right now, that was a nightmare even though we did everything to make it as comfortable as possible, it’s never going to be comfortable if you got severe M.E as we know. So I have been working with the Tachycardia myself.
However, recently the missed heart beats were new, and too often the paramedics came out, but they take so long, the missing heart beats had stopped bu the time they got here, but I was in Tachycardia, and, my blood oxygen did drop slightly while they were here, they wa Ted me to go to A & E I said no, I’m used to Tachycardia, anyway after speaking to a doctor we agreed for me to stay at home and take it from there.
However, since I have been having these night sweats waking up, checking HR and found my blod oxygen keeps dropping low. So I am trying to figure out why, I am writing to my doctor this week to explain it to her, as she is aware of my situation re M.E and doesnt know I had paramedics out, so I will wrote and let her know and then see what she wants to do. We usually discuss options.
I am aware anaemia can be a issue. I only had bloods done a month ago, all ok, although I am not sure what she tested for, so maybe that will be her approach when I contact her. More blood tests. I’d be suprosed if I am low on anything like iron, or anaemic as I tend to have regular blod tests and they all been ok, I think she tested for anaemia couple of years ago when the Tachycardia started.
Its baffling, like everything else with M.E. as its stands, all I know is when I get a sudden sweating episode my blood oxygen drops a bit. Which is called hypoxemia, but not officially diagnosed, and my oxemetre seems accurate as paramedics said it was spot on with theirs, and I have a back up one saying same thing. I know it is adviced to get help if it drops below 95, but it’s like everything with M.E we get strange symptoms that are M.E related, like I think my tachycardia is, and not heart related as such.
I think the issue for me, is not being well enough to have tests. I mean I could never have a stress test, exercise 5o see what my o2 levels are, or anything else. When I had echo it was just regular echo no stress test, so while the ebart seems normal, we dont know what it is like under stress, exercise, neither do i know how my breathing would be under stress. How can we test these things when I have severe M.E, so hard isnt it, this M.E, because your too I’ll to go and have thorough tests.
M.E. makes everything more difficult, it’s true. You are doing good with getting testing, much better than me. Yes, it doesn’t seem that you have anemia, I am sure your doctor would have found it, she seems very careful. You are fortunate to find such a good doctor.
I think I should get an oximeter. I thought they would be expensive, but I just looked at them, and they aren’t. Which brand would you recommend?
So an oximeter can tell you if you have hypoxemia but not whether you have hypoxia? Is that correct?
I’m not sure I’m getting tested for anything. All I know is usually when I get bad, hence paramedics come out, my doctor will discuss options. When I got Tachycardia couple of years ago, I was taken into A& E and it was horrendous, and waste of time, because blood test wer normal, and ECG didnt catch it, so they send you home then it’s up to Gp. She wanted me to have echo, reluctantly due to how A& E made me worse, we waited for me to recover, took months, then I went for echo, I’ve not recovered properly from that, twice in a year was too much. Hence, why next step going to have heart monitor fitted which would mean two trips to hospital, I’ve not chased up. Me and doc spoke about it recently, after I told her i had not heard about heart monitor but I said I’m not chasing it, dont think I’d cope with hospital visit, as I was not back to my normal levels, I told her probably the M.E as Tachycardia been going on for 2 years and I’ve not had heart attack.
However, the missing heart beats which happened recently, were new symptom, started couple of weeks ago, so I rang 111 for advise, and paramedics came out, wanting to take me to A& E I didnt go, had to explain, it’s likely M.E they dont know about M.E, but as missing heart beats seemed to settle, and I was only Tachycardia by time they came, I didnt go to A& E.
However, my own doctor isnt aware paramedics came out, another doctor spoke to them, and then sent me message to make appointment with my own Gp. Which i havent yet, been recovering from paramedics. So, now i am going to write to my own Gp, tell her about missing heart beats, and the paramedics coming out. But also these sweating episodes and the dropping of blood oxygen. I assume she will test my bloods, she may not, as I had them done a month ago, but she might if there is something that can cause low blood oxygen that wasnt tested in the last blood tests, as I said last blood tests was more routine, so she may not have tested for iron or anaemia, but I was tested for anaemia two years ago, and was fine.
Yes oximetre gives heart rate and blood oxygen. Blood oxygen should be 95 to 100. Below that its called hypoxemia. Which means not enough oxygen in the blood, whereas this article is talking more about hypoxia which is the inability to get oxygenated blood to the muscles. I mention my situation because I wondered of anyone else has hypoxemia, because of there isnt enough oxygen in the blood to start with then I’d assume that would also result in hypoxia, not getting enough in muscles. Although I dont know for sure, but it makes sense. However, this article is talking about other reasons they are finding hypoxia in M.E patients.
However, for me its about finding out why my blood oxygen is dropping, is it something to do with my heart, or is it M.E causing some weird issue that is affecting my ability to get oxygen in my blood? Who flipping knows.
With regards to oximetre, many are not reliable, it took a few attempts for me to find a reliable one, I got it of Amazon it’s called Salter PX 100 EU. Hope this helps.
Sleep apnea and shallow breathing may be a contributing factor here as might be high glycolisation of thr blood.
But I agree, snthg different I’d happening
Although I say this happens at night, it’s not necessarily when I am asleep, but has also happened when I am in bed at night resting.
I never heard of high glycolisation, I will research it, from bit I just read its seems a bit complicated so I am going to have to take my time to try and understand it. As I said in my earlier losts I am writing to my doctor and will no doubt speak with her in the next week, so i can mention this, so thank you.
The investigation continues, like everything else with M.E
Have you been tested for POTS or mitral valve prolapse or any other autonomic testing? Just curious because I also have the tachycardia and the skipping heartbeat feelings and diagnosed with both after getting long Covid. I know POTS at least is a super common comorbidity with long Covid
If POTS is tested via tilt table test I couodnt do that, I’m severely affected and totally homebound, and I cannot cope with hospital visits or such things. I do know my blood pressure doesnt rise on standing, so I would say no I dont have pots. I tend to be on the lower side of blood pressure, well I used to be.
I cannot go for testing at hospital, I would only go to hospital if it was looking like real emergency. I did go once and I never fully recovered and that was after 18 months. So, going again I just couldnt manage it.
Sorry to hear about long covid. Fortunately I’ve never had covid, but neither have I’ve been vaccinated, I havent had a virus in 13 years, the last one I got was when I got M.E.
My autonomic nervous system plays a huge role in my M.E as does my immune system. My doc think the M.E hence autonomic nervous system is probably causing tachycardia, and no doubt the missing heart beats that I just started having, two years after starting with Tachycardia. However, I’m still trying to find out what is triggering hypoxemia, drop in blood oxygen.
Not sure what tests there are for ‘autonomic testing’
POTS is diagnosed based on rise in heart rate (not blood pressure).
It can be diagnosed via Active Stand Test or NASA Lean Test (which could be done in your home, if your GP was willing, or at the GP Practice, if getting there is safely doable for you).
It might be helpful to check out the POTS UK website — they’ve got lots of helpful information + a guide for GPs. Obviously ignore any mention of exercise (it helps *some* people with POTS, but is definitely not safe for the ME cohort).
I do know my heart rate didnt particularly increase when I sat up and then stood up when paramedics had me wired up. So I that sense I wouodnt say I have POTS.
I’ve developed swollen ankle now, new symptom. I guess so long of inactivity was always going to catch up with me. Trying to figure out what might be causing that. See what doctor says.
I will stay positive and do what I ca to combat these issues, but when your severely affected it’s so hard isnt it.
We need research to find out what the heck is going on and try and find a cure or some proper treatment. Not sure it will happen in my life time
I also ended up in emergency due to severe tachycardia and brachicardia (simultaneously), low oxygen and BP issues last year. It was my POTS, which was triggering kidney malfunction. You can test for POTS at home. I learned I can mostly manage it if I have 3-4L of water (including some electrolytes) per day.
Check out Buteyko s breathing methods and research about how lack of CO2 contributes to low tissue oxygenation.
“Shallow”breathing is actually what we need, belly and nose breathing exclusively. By breathing too slowly or taking big breaths we exhale too much co2.
Worth reading about his research and breathing technique.
Actually it’s the only one science backed and recommended for MECFS, too
Is there anywhere in the UK or Ireland that can do a blood test for me that will prove beyond a shadow of doubt that I have Long Covid?
Thank you
We now know that there are problems with blood flow, red blood cells and nutrients (?) etc… to get into the tissue. The question now arises: can the ANS act as the primary cause to start this cascade? Or does the ANS react with an overactive response? (as a distress signal) to these shortages due to the lack of oxygen etc…? We are leaving the immune system out of consideration for now.
But think..why would the ans be so trigger happy. Plenty of people with copd that don’t have that fight or flight.
It’s phenotypical
I became ill in 2010 and was diagnosed with POTS @ Mayo Clinic via a tilt table test soon after. I was diagnosed with ME/CFS by Dr. David Kaufman in 2014.
Dr. Kaufman opened the Center for Complex Diseases and is a working member of the U.S. ME/CFS Clinical Coalition and part of the Guideline Committee. He helped write the Consensus Recommendations published in the Mayo Clinic proceedings.
I also had a CPET Cardio Pulmonary Exercise Test that showed my level of severity was moderate to severe. I was homebound and much of that time I was bed bound also.
I worked with Dr. K for 6 years and under went tons of testing and a multitudes of off label medications, supplements and treatment trials. There were 2 things that moved the needle a little for me. The first was an antiviral and the second was fludrocortisone. The antiviral gave me the ability to be out of bed 6-8 hours a day-took me from functioning at around 25% to 35%. The fludrocortisone enabled me to have 2-3 hour outings a few days a week-guesstimate it took me from 35% to 45-50%. I still suffered with PEM.
In 2017 I first heard about Nervous System Dys-regulation and that some were saying it could be at the root of ME. I worked as an RN prior to becoming disabled by this illness. When I began to hear that people with ME?CFS were recovering through addressing this dysfunction of their nervous systems, here were just some of my thoughts…
That is absolutely ridiculous. They obviously did not have ME. They definitely were not as severely ill as I am. This illness is not in my head-my body is broken!
Fast forward to 2022 I came across Raelan Agle’s YouTube channel and began listening to some of the recovery interviews she had done. From their stories, these individuals sounded to be very ill like myself with many the diagnosis like myself…
ME/CFS, MCAS, POTS, Small Fiber Neuropathy, Mold Illness, CDC positive Lyme, Sjogrens…
After listening to several of these interviews I thought…I have nothing to lose by trying. I put my life on hold as I focused on this process of rehabilitation. It took me 14 months to final say to myself, I am fully recovered. The work was tedious and one of the hardest things I’ve ever done!
But I am living an incredibly full life now without any symptoms and absolutely no PEM. I eat what I want. I socialize as much as I want. I’m physically active. I have taken pickleball lessons and might have a slight addiction to it. I play 3-4 times a week and I’m learning line dancing, riding my bike, walking 3-4 miles, hiking…
I did not believe I could have this life after being so severely ill for so many years. I’m have so much gratitude for each and every day!
If anyone is interested or wants more information you can listen to some recovery stories on Raelan Agle channel and go from there. She has 225+ recovery videos and is adding to them several times a week.
There are many recovery programs now but also a ton of free content available.
I wish recovery for every single person that is suffering from ME and the multitude of diagnosis it brings with it.
I highly recommend an amazing book by Jan Rothney-Breaking Free. This book helped me understand what had happened to my body, why I remained so incredibly ill for so many years and how to begin to recover.
This illness is not just in our head. It is absolutely physical! Our bodies can no longer find homeostasis but as I gave my mind and body what is needed I began to slowly recover.
Our nervous systems affects every signal organ and systems in our bodies via the vagus nerve. I now know that just living with this illness and all that came with it caused me to live in a persistent state of fight or flight and this cause me to live for so many years in a state of illness. This was not the onset of my Illness. My trigger was a virus. The straw that broke the camels back so to say.
Like you, Brenda, I had a viral onset over three decades ago and became aware of neuroplastic “brain retraining” about five years ago through Dan Neuffer and then Raelan Agle. I also decided to give it a try and did the Re-Origin program for almost a full year, but didn’t experience any improvement. After that, I tried lab neurofeedback (another form of brain retraining), which was successful in reducing anxiety that came on after my COVID vaccinations. All that to say, I believe it can work, but it may not be a silver bullet for everyone. Dan Neuffer says most folks who “recover” (everyone has their own definition of what recovery is!) have done more than one thing (and that brain retraining is often one of those things). I’d be interested to hear more about your experience, if you are willing to share. srm3555@telus.net
Scott thank you for your response and I will share a bit more regarding how I address the dysfunction of my NS. the next couple of days are busy and I might not get back to you for a day or two. Would you like me to respond here or in an email to you?
Thanks Brenda, whenever is convenient; no rush. Please PM via email and we can always switch to telephone later if that’s less cumbersome. Cheers
I too would also be interested in if you signed up for a particular course to help you learn how to regulate your ANS. I’ve been watching Raelan Agles recovery stories. I understand it but I just don’t have a plan without signing up for someone’s course—I understand that there’s a few good ones. But can one do this in their own? Thank you!
Yes, this is what I would like to know. My HCP (health care professional) says low oxygen in the brain is enough to cause sympathetic overdrive.
It is interesting to see Dr. Systrom reconfirming what Dr. Paul Cheney knew years ago…ME/CFS patients have left ventricular heart failure which means that the blood leaving the heart during exercise doesn’t return rapidly enough. (Or as Systrom puts it low ventricular filling pressures.) Dr. Cheney studied this extensively with heart ultrasound procedures every time you had your annual visit. As my husband put it, I had enough energy to “get there” but couldn’t get back.
Why is this happening? We won’t ever know until scientists start looking for causes, not outcomes.
As an aside, heart problems in Long Covid and ME/CFS are not at all the same. I had the left ventricular heart problems during my 40 years with ME/CFS, but Long Covid caused A-Fib and high blood pressure. The latter occurred two months after I thought I had recovered from Covid. I couldn’t do anything without my heart rate going crazy. Fortunately, this has mostly resolved, but I have been left with balance and walking difficulties I never had with ME/CFS.
I’ve been living with ME/CFS since 1991 and, like you, have had heart issues up the kuzoo. I recovered from my second go around with Covid the end of May. Last week I suffered an experience like no other in all the years since ME/CFS…a heart rate that skyrocketed to 200 bpm precipitated by a blast of adrenaline that came out of nowhere. I was sitting perfectly still enjoying a cold drink. It lifted me off my chair and I crashed onto the floor fighting to stay conscious. Did your heart rate issues resolve naturally, or did you seek out medical intervention? I can’t go through that ever again. I was blindsided by the whole thing.
I would be interested to know how if May Turner (iliac vein compression) is enough to prevent the blood returning to the heart (cause preload failure). I do not see any literature suggesting that it is. I’ve been thinking about getting a stent at MIPS though I am concerned it will cause more issues than it fixes. I have MECFS but they indicated that half of long Covid patients improved with a stent.
Hi
Its weird cos I play golf with me
Its near kills me but I need it
I have to play always without food….go on no fuel at all.
At least 50 time I have eaten in round and heart rate doubles and I’m ready to collapse.
If I dont eat HR stay at 60 to 70 and I can survive 18 holes with some pain.
Figure that.
BTW I walk no buggy.
Its 17k steps.
I pay next day bad.
But life is too short.
I think that might be both blood volume reducing and going to the stomach and mast cells rea ting to the food and compromising blood flow
Hi
Yes could.be mast cells and cell leakiness post food.
Or blood flow diversion to gut.
Thank you for a nice summary, Cort.
This doesn’t really give me hope for recovery or a cure. Seems like a lot of irreparable damage. Am I wrong?
I have no idea if the damage is irreparable or not – actually I assume that it’s not actually because some people do get well. What encourages are findings like these could help explain why people’s functionality gets hit so hard…
Something pretty significant is going to have to happen to cause that so I am glad to see various ways that could stop oxygen/nutrients from flowing to the muscles.
It may also be that one thing leads to another; that is perhaps these problems start in one place and then it morphs from there. If that’s the case maybe you just need to get to that one thing….
Thanks Cort. I have to keep reminding myself that people do get better. Glad to see these European labs doing such excellent work.
Keep saying it. Regenerative medicine abd crispr are the ways out of this
Do people get well? From what? Since we don’t have a test for ME/CFS, how do we know what they got well from or if they stayed well?
During my years of treatment with Dr. Cheney, I had what he termed a functional cure for some time. I think he called it a functional cure, because he knew the underlying problem wasn’t going away.
And it came roaring back after I got Covid.
I don’t want to take away people’s hope, but we are no nearer to knowing the cause of ME/CFS than we ever were so how can we talk about cures?
Obviously we’d like to know how this all started but there are hundreds of recovery stories on the internet! Health Rising has 70 or so and we just added two s- and most of them are quite different from each other. All we can say is that symptomatically they looked like ME/CFS and clearly quite a few subsets exist.
Hi Cort, Not to be argumentative, but 70 cases of self-proclaimed cures and hundreds (?) on the internet don’t give the 3,300,000 million Americans with ME/CFS a lot of hope. And how about the 20,000,000 with Long Covid.
I have had ME/CFS more than 40 years and while I have had remissions. I am not cured.
I don’t think we can get excited or even hopeful about a cure claim unless it can be replicated in thousands of those with these conditions.
I hate to be a broken record, but this is what happens when you don’t know the cause.
There’s one place this is very evident – some of us, like myself, have Epithelial Basement Membrane disorder in the cornea (also known as Map Dot Fingerprint, named for the wrinkling). I wish this article had some hints of possible treatments or helpful supplements.
I have to say I find it rather astonishing that NOT ONE of these speakers mentioned fibrinaloid microclots as the cause of trouble, for which @resiapretorius and I have published EXTENSIVE AND SELF-CONSISTENT EVIDENCE. A summary with links is at http://dbkgroup.org/longcovid/. Oh, and collagen is well known to be amyloidogenic.
I was a bit surprised by that as well and actually I forgot to put it in there as a potential barrier to blood flows – and I will.
Thanks for the tip on collagen and amyloids 🙂
My gut tells me Dr. Bruce Patterson should see some of this work, if he hasn’t already. If I understand correctly, circulatory deficits were an end result of the immune dysfunction he and his team have been researching. Perhaps you could ask him for his perspectives, through the lens of his own work, Cort?
Not the worst (and we are compiling a list) but type 1A scores 0.73 at AmyloGram http://biongram.biotech.uni.wroc.pl/AmyloGram/ which is definitely in the strongly amyloidogenic territory.
I have been taking collagen powder for a few weeks and thought I would do something good because of the increasing weakness of my connective tissue. Now this article!
Are there any answers to the question of whether I am harming myself by taking collagen or does the intake have nothing to do with the process of deposition because there may be two different processes?
I would like to know this also. I’ve been taking collagen powder for a year…..so, should I stop?
Thanks, Cort..
They’re not related in my opinion but collagen won’t harm you unless you’re sensitive to it
Cort, thank you so much for summarizing the conference!
🙂
W/r/t In 2021, Bloch:“The oxygen dissociation curve of blood in COVID-19“, which, if I have it right, demonstrated an increased affinity of oxygen in hemoglobin in COVID-19. This suggests that oxygen is binding very tightly to hemoglobin (probably caused by increased levels of methemoglobin)
Anyone have anything on cytochrome b5 reductase?
Methemoglobin Reductase:
The enzyme cytochrome b5 reductase normally converts methemoglobin back to hemoglobin, keeping its levels low.
Cytochrome b5 reductase
Cytochrome b5 reductase (c5br) is a NADH-dependent enzyme known as a flavoprotein that results in the chemical reduction to two different isoforms, a soluble form and a membrane-bound form.[2] This enzyme is involved in the transfer of reducing equivalents from NADH due to the FAD electron acceptor in cytochrome b5, located in complex III of the electron transport chain, which results in the two isoforms due to alternative splicing. The overall reduction reaction from cytochrome b5 reductase aids in the control of iron in red blood cells, which dictates the amount of oxygen cells carry.[3]
I don’t remember reading anything about hypoxia related to iron in the article.
Also, folate and B12, and their relationship to low iron levels, seems like something to consider.
Folate deficiency can play a role in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), and supplementation, especially in combination with vitamin B12, may be beneficial for some individuals. Studies have shown that a significant portion of ME/CFS patients have low folate levels, and addressing this deficiency, potentially through B12/folate injections or oral supplementation, may improve symptoms.
Here’s a study w/r/t iron dysregulation in people with rolonged ill health following severe acute respiratory syndrome coronavirus 2.
https://www.nature.com/articles/s41590-024-01754-8
In the absence of any discussion of Prof. Resia Pretorius’ work and the multiple papers that she & Prof Doug Kell have published on the subject of Fibrin Amyloid microclots, clogged capillaries and the consequent impairment of oxygen perfusion of tissues, this discussion is deficient and incomplete.
https://www.resiapretorius.net/research.html
I am somewhat disappointed that hardly any of the presentations attempted to explain how the respective findings may explain PEM. After all, PEM defines ME/CFS – if you can´t explain PEM you don´t explain ME/CFS…
Nevertheless, this is exciting stuff.
Dear Annette, I am so sorry to read about your very frightening heart symptoms. I was fortunate to find a wonderful non-interventional cardiologist who is a professor of medicine at our local university medical school. He has monitored my heart for about a year and things have gotten much better. No spikes of Af-B and my blood pressure has come down. I take 400 mg. of CoQ10 which I believe has helped, but no heart medications.
I still have the left ventricular dysfunction that I have always had with ME/CFS, but I can control that with careful pacing. My Galaxy watch is an excellent way to know if my heart rate is over what it should be.
When I was at the worst of the Covid heart problems, my watch rang an alarm and called 911. I had no idea it could do that. I recommend some kind of monitoring device like my watch. They also have a new pacing monitor just for people with ME/CFS and/or Long Covid.
What I would like to know now, a matter for follow up research, is WHY are there collagen deposits?
You mentioned excitement over Rituximab at the conference. Is there somewhere I can read more about that?
This is maybe wild, but I’ve been thinking about the blood flow issues, oxygen diffusion issues, etc. that seem to be present in CFS/ME, and the issues with flexibility of red blood cells (RBC)/erythrocyte deformability, and I started looking into supplements or meds that specifically increase RBC deformability. Some things that showed up were omega-3s, L-carnitine, picroside II (extracted from Picrorhiza kurroa), and zinc.
One medication that showed up was Naftidrofuryl, see section 4.2 in https://pmc.ncbi.nlm.nih.gov/articles/PMC9415189/ (quotes at bottom of comment, to make skippable)
It’s referred to as a nootropic in this paper, but seems to be most commonly used for intermittent claudication, which “… (IC) typically refers to lower extremity skeletal muscle pain that occurs during exercise. IC presents when there is insufficient oxygen delivery to meet the metabolic requirements of the skeletal muscles.” https://www.ncbi.nlm.nih.gov/sites/books/NBK430778/
I saw this post Cort made ages ago in 2010 about intermittent claudication: https://forums.phoenixrising.me/threads/claudication-peripheral-artery-disease-and-cfs.2923/ , which is the only overlap I’ve been able to find, essentially saying the description seemed similar to
It doesn’t look like anyone has looked into this drug for CFS/ME treatment, but I’m really interested in its ability to improve RBC deformability and to potentially help prevent the decrease in ATP levels typically seen with a lack of oxygen (hypoxia) in tissues. It also seems to improve blood circulation without necessarily impacting blood pressure too much (I read this somewhere, can’t find citation, looked through effects + side effects and can’t find a mention of blood pressure which seems like evidence that it probably doesn’t effect it much, but).
“Naftidrofuryl in vitro has shown a regulatory impact on deoxyglucose uptake [122] and glucose utilization [123], and it inhibited the hypoxia-induced decrease in ATP levels in fibroblasts and endothelial cells in vitro [124].”
“In a double-blind study in human volunteers, naftidrofuryl increased erythrocyte deformability and flow [132]. The induced reduction in the lactate/pyruvate ratio in healthy human volunteers during exercise suggests that naftidrofuryl increases the efficiency of aerobic metabolism in oxygen-deprived tissues [133]. It also has a positive effect on the energy metabolism of the neuron”
above quotes from https://pmc.ncbi.nlm.nih.gov/articles/PMC9415189/
Thank you for the excellent summary of the conference findings! I’m a researcher in social sciences, so I’m always asking questions! My question these days, as I try to manage my ME/CFS with pacing and supplements and now neuroplasticity, is what is the role of a disregulated ANS in causing all these issues? How does brain retraining fit into this physiologically complex picture? We can all benefit, I think, from neuroplasticity, but how much effort should I put in? My problems seemed to start post HELLP a syndrome and get worse post-virus in 2008.
There is no bottom line answer unfortunately. Some people do really well with brain retraining and personally I think everyone should give it a go given the many positive reports. If you do it, the only thing I would suggest is to do it fully as the people who benefit really seem to engage with it.
As to the ANS – it’s a clearly a core problem which can affect everything from the stress response to blood flows to the immune system. How much of a role it plays no one knows. I do suspect that an upregulated stress response plays a major role….how to calm it down is the question Brain retraining certainly works for some people and works very well.
Thanks, Cort. I appreciate your thoughtful response. I’ll give brain training a good try!
On a different note, I just had a bout of DVT and PE with no obvious risk factors. I can’t help but wonder if there’s a connection to ME/CFS or POTS. A family friend with CFS died of the same (clot), so am feeling blessed to have caught it early.
Definitely worth a try. Good luck with that!
Wow- big clot problem! Thank goodness you caught that early. Certainly microclots have been a big deal in long COVID and ME/CFS (which suggests POTS as well. I was surprised at how well the clotting treatments did in the TreatME survey. I’m going to have a blog on a guy who’s been creating natural anticoagulants.
‘In conclusion, it appears that multiple barriers to blood flows and the diffusion of oxygen/nutrients to the tissues (not to mention the removal of waste) are present in these diseases. They include thickened capillaries and muscle fibers, reduced capillary density, endothelial cells with narrowed or even blocked passages for blood flow, deformed and stiffened red blood cells, and blood cells that are tightly clinging to their oxygen molecules.’
This is very interesting.
Does this mean we should avoid collagen supplements?
Using hydrolyzed proteine with high collagen.