

Geoff’s Narrations
The GIST
The Blog
How quickly things can turn. Suddenly, fibromyalgia, the forgotten “complex chronic illness (CCI)”, is making waves – and big ones. Move over ME/CFS and long COVID – the biggest news in the CCI world might just be coming from their mostly forgotten cousin – fibromyalgia.
Talk about laying it on the line! The authors start off the recent preprint, “The sensitizing effect of IgG in fibromyalgia syndrome is mediated by Mrgprb2 in mast cells“, with an indisputable fact: despite all the work on the central nervous system in FM, the FDA-approved drugs targeting the central nervous system have largely failed. (It’s kind of ironic…FM has 3 FDA-approved drugs, ME/CFS has none, and FM isn’t that much further along treatment-wise than ME/CFS.)
“While many patients may experience modest improvements with light exercise, recommended medication treatment with low-dose tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors is effective in only a minority of patients and often drug tolerance develops. This highlights the critical need for new, effective therapeutic strategies informed by a deeper understanding of the underlying pathophysiology of FMS.”
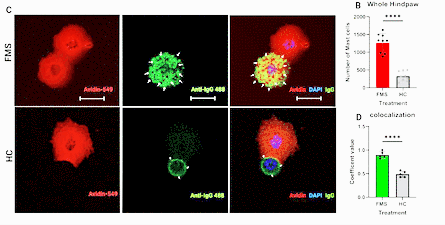
Effects of FM IgG on top / healthy control IgG on bottom. Note how the FM IgG is binding to the mast cells at top (green arrows).
The GIST
- Suddenly, fibromyalgia, the forgotten “complex chronic illness (CCI)”, is making waves and big ones. Move over ME/CFS and long COVID – the biggest news in the CCI world might just be coming from their mostly forgotten cousin – fibromyalgia.
- The spike in interest began when Andreas Goebel was able to largely replicate fibromyalgia symptoms in mice by exposing them to IgG antibodies from fibromyalgia patients.
- The antibodies gathered on the glial cells covering a key sensory hub leading to the spinal cord – the dorsal root ganglia. The question remained, though, how the antibodies were turning on the glial cells and ultimately the dorsal root ganglia and, in the process, slamming the central nervous systems of FM patients with pain and sensory signals.
- The latest study found that connective tissue mast cells were binding to the FM patients IgG antibodies. When these mast cells were disabled by removing a receptor which the antibodies use to turn them on, the mast cells settled down and the mice stopped exhibiting pain.
- Next they found that human cells treated with FMS-IgG resulted in increased secretion of a key immune factor involved in chronic pain called IL-6 . Validating work done almost 30 years ago, they doubled down on the FM/mast cell connection when they found that the skin of people with FM had increased mast cell density (as well as increased intra- and extra-cellular tryptase.)
- This suggested that treatments that can reduce mast activation by blocking a specific receptor called MRGPRX2/b2 might turn this process off. While no drugs are FDA approved to do that now several are under development. Other mast cell treatments are available and the TREATME survey suggested that some may be quite helpful.
- IL-6 blockers are another possibility. Several are on the market now and many more are underdevelopment. One small IL-6 blocking trial suggested that IL-6 blockage may be helpful.
- All in all, this is a rare study that purports to possibly be getting at core biological mechanisms that are producing pain, fatigue, gut issues, and other problems in a considerable number of fibromyalgia patients. Finding a problem receptor on a cell is potentially big news in any disease, as turning it off can stop a disease process in its tracks.
- Since problems found in FM could translate to ME/CFS and/or long COVID, the finding is of interest in these diseases as well.
The big question now is whether “a deeper understanding” has finally been achieved. The field is certainly acting as if it may have been. Goebel’s initial finding that IgG antibodies are ramping up pain levels by tweaking the satellite glial cells on the dorsal root ganglia in mice has triggered a bevy of studies. With seven studies/papers published this far, the FM field hasn’t been this excited about anything since small fiber neuropathy popped up.
It’s no surprise. Thus far, the fibromyalgia antibodies have been shown to produce in rodents “profound” increases in pain sensitivity, reduced movement, decreased grip strength, gut issues, and reduced small nerve fiber density in the skin.
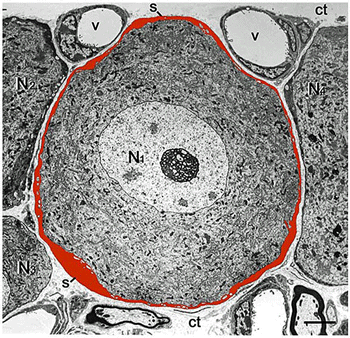
Satellite glial cell (red) surrounding sensory neuron. (Image -Hanari-CC-40.-Wikimedia_Commons)
Plus, in more severe FM patients, increased binding of IgG to the satellite glial cells surrounding the sensory nerve bodies in the dorsal root ganglia (DRG) correlates with pain intensity. If one wanted to send the central nervous system into a tizzy by amping up pain and sensory signals coming from the body, the DRG would probably be the first spot in the body to target.
While it’s not clear that this process is happening in all FM patients, it all fits very well. The question remains, though, how this process is being accomplished.
It appears that the IgG antibodies have clustered themselves on the satellite glial cells covering the dorsal root ganglia. We know that hyperactive satellite glial cells have been implicated in chronic pain. What we don’t know – and what this paper purports to uncover – is the exact mechanism through which the IgG antibodies from FM patients are tweaking those glial cells and causing pain.
Back to the Study
“Our findings indicate that FMS-IgG has unique properties that promote the activation of connective tissue mast cells (CTMC).” the authors
The UK/Johns Hopkins group immediately went to mast cells to potentially explain the pain hypersensitivity. First, they validated the increased connective tissue mast cell skin levels found in fibromyalgia back in 1997. Next, they showed that the dorsal root ganglia were going gangbusters – ratcheting up pain levels.
Then they showed that increased IgG antibody binding to the mast cells was present, suggesting that antibodies in FM patients were activating the mast cells. When mice lacking a mast cell receptor called MRGPRX2 didn’t respond negatively to the FM patients’ antibodies, and mast cell levels remained normal, they concluded that antibodies were ramping up the mast cell activity. The researchers also found evidence that the FM antibodies were causing gut distress.
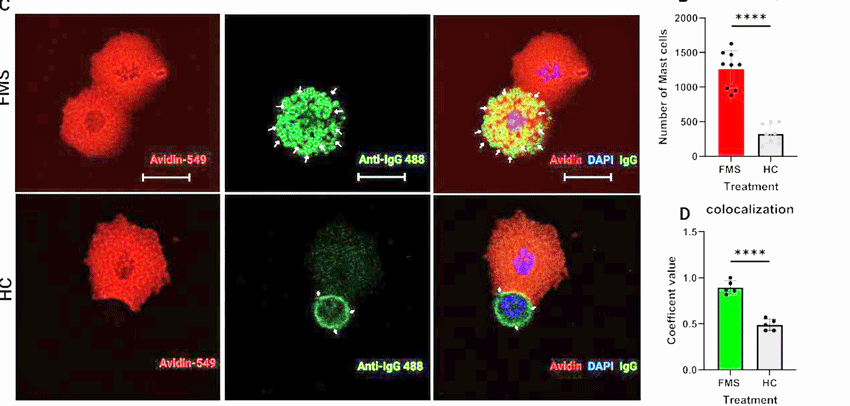
Effects of FM IgG on mast cell binding. (FM IgG on top/ healthy control on bottom.) Note how much the FM IgG is binding to the mast cells at the top (green arrows) while the HC IgG is not (bottom). Also notice the huge difference seen in the graph (red) at the right.
When FM antibodies increased the production of IL-6 levels in human cells and histamine, tryptase levels were found to increase in FM skin cells; the deal was pretty much sealed.
Mast cells commonly emit histamine and tryptase, but the IL-6 finding was really something. IL-6 upregulates histamine production, and chronic IL-6 exposure causes mast cells to become hypersensitive to stimuli. If that wasn’t enough, IL-6 actually helps mast cells survive when they are exposed to low oxygen conditions.
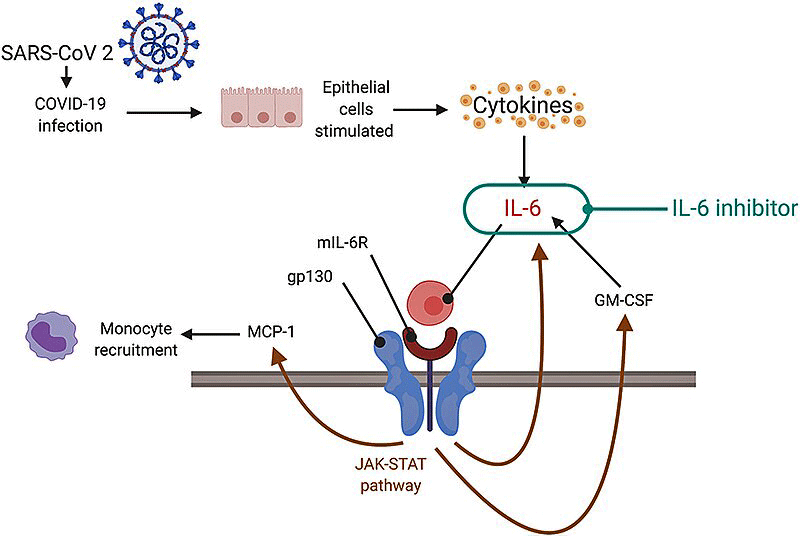
IL-6 plays a key role in the cytokine storms caused by the coronavirus. Note how an IL-6 inhibitor, called a JAK-STAT inhibitor, attempts to calm the storm down. Wes Ely’s REVERSE LC trial, funded by the NIH, is employing a JAK inhibitor. (Image from
IL-6 is particularly important because it may be ground zero in the cytokine network when it comes to producing chronic pain. IL-6 hypes up the activity of the sensory and pain-producing nerves, is implicated in neuropathic and inflammatory pain, activates pain-enhancing pathways in the central nervous system, turns on the microglial cells (neuroinflammation), and, as we’ve seen, buddies up nicely with the mast cells.
In general, fibromyalgia studies have found increased IL-6 levels, and it’s believed to be a significant contributing factor.
The authors noted that other factors could be turning IL-6 in FM as well, but this was the first study suggesting that ndicated that mast cells were.
Earlier, using a similar passive transfer technique, the group implicated IgG antibodies in the torturous complex regional pain syndrome (CRPS). This study, though, showed that IgG antibodies were not turning on mast cells in CRPS, and some other IgG-related process was occurring. Apparently, IgG antibodies can produce chronic pain in several ways.
Fibromyalgia: A Mast Cell-Connective Tissue Disease?
We generally don’t think of fibromyalgia in terms of the immune system, let alone in terms of the rather dodgy and difficult-to-study mast cells. Over time, however, about a dozen studies and papers have examined mast cells in FM. In fact, despite the interest in mast cells in ME/CFS, there’s more direct evidence that mast cells are dysregulated in FM than there is in ME/CFS.
This is because increased mast cells have been found in the skin of FM patients at least three times. The first – back in 1990 – from Sweden, suggested, get this, that IgG binding caused by mast cells to the extracellular matrix (read collagen – read connective tissues) was hampering capillary blood flows (!). That’s very close to the Wust group’s finding that the extracellular matrix problems are reducing capillary blood flows in ME/CFS, and predates the mast cell-connective tissue discussion on these diseases by about 30 years.
The Swedish group extended their findings in 1997 by finding higher IgG deposits in the skin and blood vessel walls, a higher reactivity to collagen (the main component in connective tissues), and higher numbers of mast cells. (These problems were not, interestingly, found in rheumatoid arthritis.) This was the last research linking mast cells to connective tissue problems in FM for almost 30 years.
The connective tissue findings, however, continued. In 1997, a German group found problems with the extracellular matrix/collagen surrounding the nerve fibers and evidence (Pyd/Dpyd ratios) of connective tissue disease in FM. They suggested that “remodeling of the extracellular matrix and collagen deposition around the nerve fibers in fibromyalgia” was contributing to the lower pain thresholds.
In 2005 and 2007, a Danish group found that reduced levels of collagen in the skin, lower levels of intramuscular collagen, which could “lower the threshold for muscle micro-injury” and evidence of dysregulated collagen metabolism. That was it for collagen findings in FM for almost 20 years.
In 2025, though, Dr. Ilene Ruhoy started off her discussion of complex chronic illnesses like ME/CFS, FM and long COVID in her book, “Invisible No More“, with sections on “Connective Tissue Dysfunction” and “Mast Cell Activity” and noted what a high concentration of mast cells are found in connective tissues. She reported that connective tissues hold every organ, blood vessel, nerve fiber and muscle in place and considers connective tissue problems a major feature of these diseases.
What about ME/CFS?
Anytime we have a finding in one of the “complex chronic diseases,” we need to look to see if it’s been replicated in the others. The evidence of dorsal root ganglia involvement in ME/CFS is anecdotal but compelling. Dorsal root degeneration and/or inflammation was found in three of four ME/CFS autopsy studies. The DRG dysregulation found was believed to contribute to the sensory issues and autonomic nervous system dysfunction each of the ME/CFS patients experienced.
Dr. Martinez-Lavin has called the DRG a “pain factory” and reported that ME/CFS patients with fibromyalgia have exercise-induced DRG-produced pain. Given that the nerve fibers that convey sensory information to the DRG are unmyelinated, the small fiber neuropathy found in the skin and corneas of fibromyalgia and ME/CF patients may be affecting the fibers that lead to the DRG in both illnesses, as well.
Interestingly, “abnormal blood supply via the capillaries, leading to the entry of inflammatory cells, proteins, and other toxins into the neurons”, is believed to play a role in DRG dysfunction/damage. This is because the capillaries that supply blood to the DRGs tend to be “leaky”, allowing inflammatory cells, proteins, and toxins to pass into the neurons.
Treatment
Potential therapeutic targets are what we want, and this study has two to suggest. There’s the MRGPRX2 receptor present on connective tissue mast cells, and there’s IL-6 – a star performer when it comes to producing chronic pain.
The Mast Cells
One of my first reactions to reading the paper was that it had to be mast cells! Why couldn’t it be the T-cells? Instead, it had to be quirky, difficult to measure, mast cells. For all the interest in mast cells at the clinical level, I could not find one study that actually assessed mast cell levels in ME/CFS. No mast cell clinical trials have been done in either ME/CFS or FM.
Mast cell treatments, of course, can be helpful – and even inexpensive – but it can take time to find what works, most doctors are unaware of them, and the effects, while helpful, tend to be moderate.
That said, the Open Medicine Foundation’s TREATME survey found that MCAS treatments were among the more commonly effective treatments. Of the 15 MCAS treatments assessed, only two were rated as moderate/much better by less than 10% of patients, four MCAS treatments were rated well by between 10% and 20% of patients, and eight by between 20% and 30% of patients.
Two were reported by over 30% of patients (ketotifen + H2RA (40%) and H1RA + H2RA (combo) (38%)) to moderately/much improve their symptoms. Note that combinations of mast cell treatments seem to work best. (Check out more in Table 3 (circled in blue) of the supporting documents, which lists the percentage of patients who moderately/much improved using mast cell inhibitors.
The list of mast cell inhibiting drugs (cromolyn, ketotifen, H1/H2 blockers, Montelukast (Singulair®), zafirlukast (Accolate®), zileuton (Zyflo®) and supplements (luteolin, quercetin, nettle, bromelain, skullcap, resveratrol) is fairly long. Other mast cell drugs approved for systemic mastocytosis (which is different from mast cell activation syndrome), such as Avapritinib (Ayvakit®), Midostaurin (Rydapt®), Imatinib (Gleevec®), do not appear to be used much. Others, such as Omalizumab (Xolair®), can be difficult to get.
If mast cell activation syndrome plays a major role in ME/CFS and allied diseases, we clearly need better tools to diagnose and study it, and better treatments for it. Some treatment help may be on the horizon. Particularly interesting are a new class of “highly selective, potent, orally active” drugs that target the MRGPRX2 receptor (Compound B/Compound A, PSB-172656, B-1023 and B-5023, EP262, EP547) that was highlighted in this study.
Other drugs include next-generation tyrosine kinase inhibitors (Masitinib, Nintedanib), a mast cell number reducer (Barzolvolimab (CDX-0159) in phase III trials), and Lirentelimab. How soon any of these might become available is, of course, unclear.
The Ultimate Target?
Note that this study suggests mast cells are an intermediary step in FM. Since the more proximal cause is the IgG antibodies that trigger them, B-cell-depleting, antibody-depleting, or antibody-regulating treatments could have a role to play. Indeed, one scenario – that the failure of the adaptive immune system results in a compensatory response from the innate immune system – suggests that treatments focused on other parts of the immune system could rein in the mast cells. On that note, IVIG was the most efficacious treatment found in the TREATME ME/CFS/Long COVID survey. With that, the 380-person IVIG RECOVER Initiative Long COVID dysautonomia study is looking more exciting all the time.
IL-6 Inhibitors
We’re on a bit stronger ground with IL-6 inhibiting drugs. While the list of approved IL-6 inhibiting drugs
(Tocilizumab (Actemra, biosimilars), Sarilumab (Kevzara), Siltuximab (Sylvant) and Satralizumab (Enspryng)) is not a long one, IL-6 inhibiting drugs also appear to be a growth field and one, Tocilizumab, has already been tested in FM. A case series of 6 patients found moderate reductions in pain (>30%) and significant improvements in energy and sleep quality after 4 to 12 weeks of treatment. A small test of an IL-6 inhibitor (anakinra) in ME/CFS failed to show benefits.
Multiple drug companies are developing a variety of IL-6 inhibiting drugs and planning to use them in combination therapies with JAK STAT inhibitors. Plus biosimilar (cheaper) versions of tocilizumab and other established IL-6 drugs are entering the market. One analysis predicted the IL-6 market will see “considerable development” over the next ten years, and stated that “the discovery of fresh IL-6 signaling functions in chronic diseases drives fast market innovations and strategic alliances.”
JAK inhibitors can also reduce IL-6 levels and a major JAK-Stat inhibtor study (baricitinib) is underway in long COVID.
https://www.healthrising.org/blog/2024/01/19/ely-long-covid-chronic-fatigue-baricitinib/
Rinvoq is another JAK inhibitor that is not currently being trialed but did produce a remarkable recovery in a very sick ME/CFS patient.
https://www.healthrising.org/blog/2024/11/22/jen-jak-inhibitors-chronic-fatigue-syndrome-long-covid/
A seeming outlier reported by Dr. Martinez-Lavin, which has not been tried in any of these diseases, is using dorsal ganglion stimulation to reduce sympathetic nervous system activity.
Conclusion
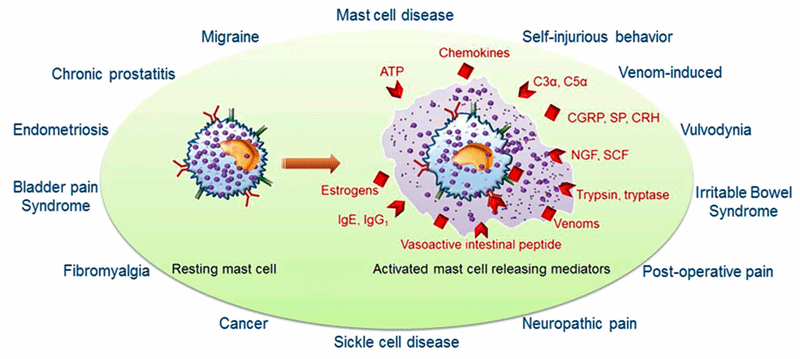
Chronic pain syndromes reportedly associated with mast cell activation. (Image from Anupam-Aich, Afrin_Kalpna-Gupta, 2015 from Wikimedia Commons)
While these findings seem to line up nicely, they are mice/human studies and need to be validated by other groups. (The senior author Xinghong Dong is the scientific founder of and consultant for Escient Pharmaceuticals, a pharmaceutical company that is developing MRGPR targeting drugs (MRGPRX2 is an MRGPR receptor. After all the problems getting the drug industry involved in ME/CFS, I was actually happy to see industry involved in this study.)
All in all, this is a rare study that purports to possibly be getting at core biological mechanisms that are producing pain, fatigue, gut issues, and other problems in a considerable number of fibromyalgia patients. Finding a problem receptor on a cell is potentially big news in any disease, as turning it off can stop a disease process in its tracks.
Since problems found in FM could translate to ME/CFS and/or long COVID, the finding is of interest in these diseases as well. A fascinating merging of these fields around the mast cell and connective tissue issues in FM may be occurring.
Time will tell! We’re not nearly done with satellite glial cell findings in FM. More on them is coming up.






Must say, i understand very little of the medical wording but have pricked up my ears on reading finding re fibromyalgia. 34yrs ago I was told I had Polymiagia rheumatica but many years later diagnosed with fibromyalgia. IgG protein is present in the blood so I would like to know if there is a connection to the trials done here. Pain has been a companion for these 34yrs and drugs have been trial after trial. Inflammation creates pain tenderness and muscle waste. I have been told the antibodies and the protein just stay in my body and a blood test is done 3mthly. I am 80yrs old now and manage well but I do hope and pray you will progress to help the many thousands who suffer with chronic pain.
Thanks for another great post Cort. I’ve been following the work of Andreas Goebel and his collaborators for a few years now and it gives me great hope to see proper scientific work being done on FM/long Covid/ME rather than the lazy approach adopted by mainstream medicine to date of it being central sensitization! It just didn’t fit my scenario I felt in my gut from day 1!
I always had some mast cell symptoms so always thought they played a prominent role, and ketotifen has helped somewhat, but still struggle with neuropathic pain daily.
Interestingly, I also developed both aquagenic and cholinergic urticaria (hives from water on skin and hives from my own sweat on my skin) after first covid infection in 2020. I’d love to be biopsied and studied by this type of research team!
When one researches these types of urticaria-the MRGPRX2 receptor pops up there as well! I wonder are there any drugs from this area that could be applied to FM patient cases? Rather than having to wait for a brand new drug…
Would also love to know the overlap between patients with chronic hives and chronic pain!
It’s too bad that there haven’t been larger trials of IL-6 drugs in fibromyalgia- so many of them are out there, and all the JAK-STAT inhibitors, it could be a real win for pharma if they did work. Especially if IL-6 is very consistently elevated in FM. I am not as optimistic about doing anything to inhibit or reduce mast cells – that seems like overkill/a bad thing and as Cort mentioned is likely not a causal factor in FM but more of a byproduct of other immune system issues. If FM is truly an autoimmune condition, as evidence seems to be pointing, we should be targeting the source of the damage (if autoantibodies are attacking DRG and that is leading to all the downstream issues/perturbations) then yes target the B cells.
Cort – the TREATME study did not include FM patients, correct?
Yes, that is my understanding. The TREATME survey included ME/CFS and long COVID patients. I hope the next survey includes people with FM 🙂
You might want to get tested for hereditary alpha tryptasemia. I just found out about this condition. 6 percent of Caucasians carry this genetic marker. High tryptase levels ravage our bodies. Only discovered in 2015 or so. Jonathan Lyons discovered this condition.
Over the past 30+ years I pushed into remission most of the conditions highlighted in the above studies: CRPS, FM, Mast Cell Activation Syndrome (MCAS) and POTS/dysautonomia, Long Covid’s twin. A few years ago, Dr. Blair Grubb found some of his POTS patients have autoimmunity but not all. MCAS researchers (Dr. Afrin and others) connect mast cell activation to pain but not everyone with FM or POTS has MCAS. And, pain was not one of my MCAS symptoms. MCAS is fairly common and easy to treat but I have yet to hear of a mast cell patient who claimed their MC treatments helped with pain. While it is common to find these co-morbid conditions with Ehlers Danlos Syndrome, a connective tissue disorder which I also have, every patient has a different combination. And I have yet to find any two patients helped by the same treatments. It took releasing a compressed nerve to heal my CRPS. I pushed FM into remission by an extended fast. But neither relieved my other conditions. 3 years ago I started sipping chloride dioxide water (CDS) throughout the day and it relieved both my interstitial cystitis and MCAS. Antihistamines had helped calm my mast cells but they weren’t as effective as CDS water. They were also dehydrating. So, although I agree all these conditions can be connected and the dorsal root has been implicated by Dr. Daniel Clauw in 1997 as indeed, part of the network of pain sensitization he discovered, I’m not sure mast cells are causing the cascade of debilitating conditions in the way described.
I’ve had FM since the 1980s before it was a recognized condition by doctors. The first signs were sharp excruciating pain with a dramatic change in my soft tissue. I developed small fatty tumors all over. Doctors discovered that I had a high sed rate as well. When FM became a recognized condition, my rheumatologist prescribed amitriptyline which was somewhat helpful for decades until I developed tachycardia. Now that I am older, I experience less pain and worse sleep. When my sleep is poor, I get a terrible pain and stiffness at the base of my skull. Most mainstream doctors including specialists do not seem to have a good understanding of FM. They only seem to know about the few routinized approved drug treatments available. After I had surgery five years ago, I developed a very painful mast cell syndrome for which I was given a muscle relaxant from the MD, but I actually successfully treated it myself with benadryl over a couple of months. What remains is that there are few recognized treatments from the established medical community.
BACKGROUND: I was diagnosed with FMS in 2004 and ME/CFS in 2006. Yes, pain, exhaustion, orthostatic intolerance and such over-sensitivity to light and sound, not to mention daft ( my shorthand for a plethora of memory problems).
QUESTION: After reading this article I’d like to know: Has anyone here tried the IL-6 dampening properties of Andrographolide? It’s apparently available from the Andrographis paniculata plant native to SE Asia. I’m researching ways to increase its bioavailability, as I understand that is a problem.
Thank you, Cort, once again, for staying on top of emerging studies and for communicating their results in such a readable way!
I took 600mg Andrographis (standardized to 10%) 2-3 x day for 4 years after a tick bite, when I suspected Lyme, as well as the diagnosed Anaplasmosis. Buhner recommended 600-1200mg 3 X day for Lyme. (It has now been proven not to work as well for Lyme as other herbs) and I was diagnosed with Fibro about 6 months after the tick bite as I had tender points all along my spine and started the Andrographis tincture about that time for the Lyme. I later switched to capsules. My other pains (rotating joint and muscle, headache) continued for 4 years but at some point the Fibro points on my back stopped hurting. I was eventually diagnosed with ME/CFS and now MCAS. I would definitely try it! I posted below on info on IL6 and it’s listed there as well as well. I always felt better on it. May start it again and see if it helps MCAS but there are other herbs listed that work better for that. Hope it works for you!
Thanks, @T Allen. Very helpful. I think I’ll give it a try.
I have no knowledge whatsoever about fibromyalgia. I am an ME/CFS patient.
I have wondered for a long time why ME/CFS is called a complex chronic disease. This curiosity grew stronger when I was criticised that I was promoting the HHV-6b hypothesis too boldly. The criticism seemingly arguing that a complex illness can’t have a “simple” pathomechanism like herpes reactivation.
So I realised that many in the ME/CFS field are quite attached to this idea of a complex ilnness.
I researched a bit and Google AI found a paper from June 2024 published in Frontiers in Immunology
https://pmc.ncbi.nlm.nih.gov/articles/PMC11180809/
that elaborates on the concept in a paragraph.
The idea that is presented is actually not about ME/CFS being a complex illness but the idea that ME/CFS has a complex pathomechanism.
It is the immunologists who believe that ME/CFS is an autoimmune illness that actually came up with this idea. So, the idea is connected directly to the idea that autoimmune processes play the most important part in the development and deterioration of the illness.
Because it is from the supposition that autoimmunity is driving the disease that a an illness model is derived that looks complex we can say that without the idea of autoimmunity as the cause of ME/CFS the idea of a complex pathomechanism isn’t supproted either.
I gave my warnings about this idea before. I don’t think that autoimmunity is the best explanation for the pathomechanism of ME/CFS that we have at the moment. The HHV-6b hypothesis is not only theoretically the better hypothesis because it is formulated clearly and simply and can be tested directly, but it is also supported by a lot more of evidence.
If HHV-6b reactivation was the explanation for the pathomechanism of ME/CFS there wouldn’t be any grounds left to call ME/CFS pathomechanism complex and derived from that ME/CFS a complex illness.
Hi Cort, It is of topic but did you see this study already? I remember i read it….. 🙂
https://www.nature.com/articles/s41591-025-03788-3
Mast cells! My husband has said I am going to kill myself trying to get better. And he is almost right.
My first symptoms of ME/CFS were reactions to all kinds of things that never bothered me before…including foods, chemicals, perfumes, even chlorine in our water. This was so severe I had to give up my job as an advertising copywriter because I could no long go into print shops to pick up work or attend “Blue Sky” meetings where everyone sat around a closed room smoking (not me).
The first medication my doctor prescribed was a form of heparin and that did help with the hyper-reactivity, but not completely.
Finally, I decided to get tested for mast cell disorder. I was positive on scratch tests for myriad chemicals, so the doctor decided to do a trail of treatment. This involved small doses of two antihistamines, Singulair and Tagamet.
The next day I woke up and my vision was blurry so I stopped everything, but my vision didn’t clear. I went to an optometrist who was extremely concerned because my eye pressure was so high. He sent me immediately to a glaucoma specialist which diagnosed narrow angle glaucoma, a fast way to lose your vision. (Tagamet can trigger narrow angle glaucoma, but who knew.)
The specialist did a laser iridotomy and things seemed better until two weeks later, we flew 13 hours to Maui to visit our daughter. When we got there, my left eye was drooping and bright red. I also had the worst headache of my life.
We sent pictures to the glaucoma specialist who became very upset and sent me to his former partner who was practicing on the island. They gave me glaucoma drops.
As soon as I returned home, I was rushed to a neuroradiologist who diagnosed a carotid cavernous fistula. This means the carotid artery has ruptured and connected to the vein behind the eye.
I went in for a 7 hour surgical procedure which involved brain surgery to separate the artery from the vein with platinum coils. (The worst part of the surgery is that you can’t move at all for two hours after the procedure.)
This is a medical emergency and can result in a stroke or the loss of vision in one eye. I seem to be going for door #2 as there is now a streak in the middle of my vision in my left eye. With two eyes open, it is okay, but I could never operate with just the left eye.
Now my glaucoma seems to be getting worse (another outcome of the CCF). They are trying to control it with drops, but, true to form I am allergic to the preservative in the eye drops. There is talk of surgery…risky business if you have had a CCF.
This has all been extremely stressful, but I brought it on myself by going for the Mast Cell testing. So my warning is be very, very careful in the treatments and tests you try. You could end up much worse.
Betty, what a trial you have been through. Your husband sounds like mine–he watches my journey with equal parts wonder and dread. It’s easy to make the wrong choices when you’re hoping to find something that will make you feel better.
Connective tissue again.
I wish heaps of money was given to this area. I think it’s the birthplace of a lot of illnesses
Just an FYI. I always go looking for natural supplements because the “drugs” always have nasty side effects. Found this on addressing IL6 issues. https://selfhack.com/blog/interleukin-6/
It is time for ME/CFS and Fibromyalgia researchers to do some serious study of TILT (Toxicant Induced Loss of Tolerance).
“Exposures to pesticides, volatile organic compounds, combustion products, and mold have previously been reported as initiators of chemical Intolerance.”
In this study https://pmc.ncbi.nlm.nih.gov/articles/PMC10660865/
researchers found that TILT may be involved in many modern illnesses including ME/CFS and other health problems frequently covered in this blog.
There is an excellent chart of these health outcomes in the study, but unfortunately, I was not able to paste it into this email. Just scan down the article to the chart. This is an important paper and I hope you will struggle through it even though it takes a while.
Cort, is there is any research going on regarding toxicants being the initiators of Fibromyalgia, ME/CFS and even Long Covid? If not, why not?
My own illness began when we had our home treated for fleas with Dursban, a product that is now off the market because of its toxicity. Dursban was also used heavily during the first Gulf War, but is no longer on the military’s list of approved pesticides.
Yes, viruses, bacteria and mycoplasma can be part of the equation as is genetic susceptibility especially in methylation and the way the body detoxifies chemicals.
As a child, if I went to Woolworths dime store after they had sprayed with pesticides, my upper lip or thumb would get numb. I guess I should have paid more attention to early symptoms of TILT.
And this is the most important fact: many of these chemicals are stored in body fat where they can continue to cause problems when they are released into the blood stream. (Women have a higher percentage of body fat; could this explain why these conditions are more prevalent in females?)
What cause the release of stored toxicants? Diets involving sudden and dramatic weight loss (why I am opposed to Keto); high temperatures; excessive aerobic exercise; too much sun exposure, etc. Many of the things can trigger PEM in ME/CFS patients.
The plot thickens! Apparently statin drugs also inhibit IL-6 – see paper by Dr. Bruce Patterson’s team:
https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1122529/full
Statins are paired with Maraviroc for their experimental treatment of long COVID and other chronic invisible illnesses.
Interesting! Thanks for that, Scot!
I have been on all the recommended drugs for fibromyalgia. I found one Dr on the net is having success with an Alzheimer’s drug for hope. It is Memotine . Plan to ask my doctor about it.