

Geoff’s Narration
The GIST
“We’re seeing things in the front of the brain, we’re seeing issues in the back of the brain, we’re seeing a lot of things in between…this is a brain-wide issue”. It’s safe to say that the ME/CFS brain is an inflamed brain.” Jarred Younger
Jarred Younger couldn’t resist. He’s usually pretty deadpan, but there it was – a big smile as he started the “New Results: The ME/CFS brain is inflamed” video. Younger made the proviso that this was a first pass of his data and that things could change, but I would be shocked if Younger talked about results he wasn’t very confident in. He certainly looked happy in this video.
Younger has been asserting that neuroinflammation was a big deal – perhaps THE big deal – in ME/CFS for over ten years. Younger has been so confident that neuroinflammation is a big deal in diseases like ME/CFS and FM that ten years ago, when he opened his new lab at the University of Alabama at Birmingham, he called it “The Neuroinflammation, Pain and Fatigue Laboratory“.
Younger has hypothesized that hypersensitive microglia in the brains of ME/CFS/FM and long-COVID patients have been pumping out inflammatory cytokines and immune factors in response to the slightest stressors, and are responsible for the fatigue, pain, and other symptoms in these diseases.
The Gist
- Younger has been asserting the neuroinflammation plays a big role in ME/CFS and related diseases for over a decade, and now with his new PET scan study, he had undeniable proof.
- PET scan studies are the gold standard when it comes to assessing neuroinflammation and with one study saying “yea” and one study saying “nea”, the question was still up in the air.
- Younger’s new study, though, which used a more advanced radiotracer found scads of neuroinflammation, enough for Younger to state that people with ME/CFS have inflamed brains.
- We’ll see that the brain in ME/CFS appears to be stuck in a kind of chronic state of post-infectious illness or “sickness behavior”. Sickness behavior refers to the symptoms the brain produces to keep us in bed in order to stop spreading an infection and to conserve our energy to fight off the bug.
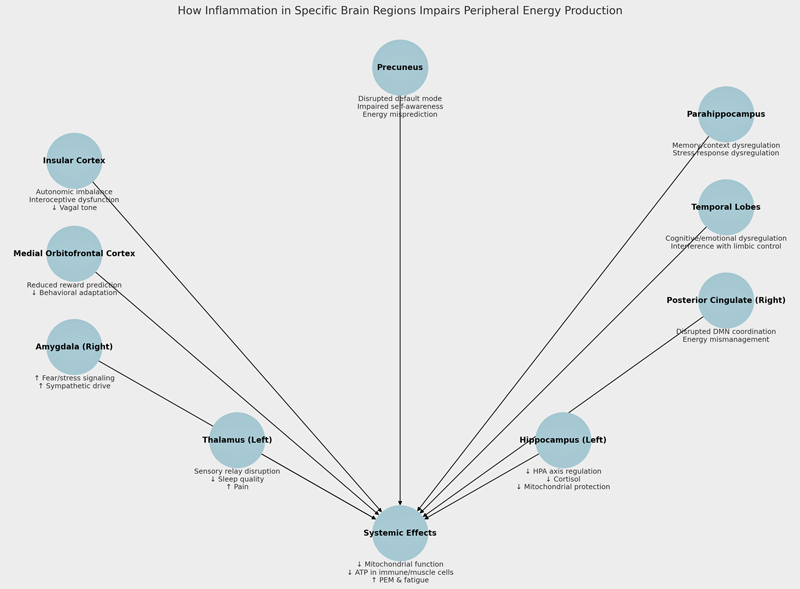
- Youngers findings, which have not been published yet, and which constitute of first pass of his data, uncovered widespread areas of “bilateral” inflammation (found on both sides of the brain) and other areas of “unilateral” inflammation.
- Bilateral inflammation is considered more significant because if only one side of the brain is inflamed, the other side can sometimes compensate for it.
- Younger said that the bilateral inflammation in the insula, alone, could produce ME/CFS. The insula is a central hub in the brain that assesses sensory and other information to determine how well the body is functioning in a process called interoception.
- An important regulator of autonomic nervous system activity, the insula is considered a “bridge” between internal bodily states and “adaptive physiological responses”; i.e., autonomic nervous system activity.
- The precuneus’s close integration with the insula, and the default mode network would likely result in difficulty in focusing one’s attention and increased feelings of fatigue, pain, and unpleasant body sensations. If it seems you’re never free from negative body sensations and have trouble getting beyond them and concentrating on other things, dysregulation of the insula, precuneus, and default mode network could be the reason why.
- While inflammation in both sides of the hippocampus produces fatigue, in the medial orbitofrontal cortex it dampens sensations of “reward and motivation”. Cognition and attention take a hit with an inflamed right medial frontal gyrus. For its part, a tweaked posterior cingulate activates the default mode network – the center of rumination – which is basically kryptonite to getting things done.
- Moving on, inflammation in the left hippocampus impacts learning and memory, but then we come to the left thalamus, which Younger called a “critically important” brain region that relays all sensory information that flows through the brain. Problems in the thalamus alone, he said, could explain ME/CFS.
- The brain Jarred Younger found, then, is a brain that seems to determine to keep us from exerting ourselves and participating in the world. It’s a brain ramps up symptoms -most especially fatigue – and body sensations – and to keep our attention focused inwards rather than outwards. A brain that’s designed to increase effort, blunt the “rewards” that typically move us, and reduce motivation.
- A hypervigilant, anxious, and unsure brain that amplifies danger and fear and, when we step over the line, whacks us with more symptoms. A cognitively impaired brain with a short attention span keeps us from engaging in the tasks we used to engage in. A brain that impairs restful sleep, which keeps us in a fatigued state.
- It’s a post-infectious, “sickness behavior” brain.
- But how to connect the inflammation found across the brain to the energy production problems in the body? I asked ChatGPT and AI Perplexity if it was possible, and both answered “yes”. ChatGPT said “inflammation in the specific brain regions you’ve listed could plausibly impair energy production in peripheral cells (e.g., immune and muscle cells). These mechanisms are highly consistent with energy metabolism abnormalities observed in conditions like ME/CFS, Long COVID, and post-viral syndromes.”
- While it’s not clear what’s causing the inflammation, note that because many of the inflamed areas of the ME/CFS brain seem to be particularly sensitive to inflammation coming from the body, inflammation is a prime suspect.
- Younger believes that neuroinflammation is what we need to be targeting in ME/CFS, stating “We have to find or develop treatments that reduce brain inflammation if we’re going to improve ME/CFS,” and that he hoped to have more “exciting news” on the clinical trials front soon.
For all the hullabaloo over neuroinflammation, though, it’s amazing how little direct evidence of widespread neuroinflammation there is. While quite a few brain studies suggest neuroinflammation is present, I could find only three papers that attempted to directly measure it across the brain.
The first attempt to directly measure it, Nakatomi’s small 2014 PET scan paper concluded that widespread neuroinflammation was present and quickly became a kind of cause celebre in the ME/CFS world. Somehow it took another seven years for a small PET scan study (n=16) to show up, but this time, using the same not-so-accurate radioactive tracer that Nakatomi did, it found no evidence of neuroinflammation.
Next, Younger’s innovative 2020 thermal mapping MRI and spectroscopy study found widespread areas of increased temperature and metabolites that were indicative of neuroinflammation.
PET scans, which highlight activated microglia, are considered the gold standard for assessing neuroinflammation. Now we had two small PET scan ME/CFS studies – one which found neuroinflammation and one which didn’t.
So while we’ve been acting, talking, and thinking as if neuroinflammation is, of course, present in ME/CFS, we haven’t directly proved that it’s present at all. With Younger’s use of a newer, more accurate radiotracer, it looks like his PET study is going to break the tie.
An Inflamed Brain
Please note that as I attempt to dig deeper into Younger’s findings, I add many things he did not discuss in the video.
Both Sides Now – Sites of Bilateral Inflammation in ME/CFS Patients’ Brains
Younger started with areas of the brain featuring bilateral inflammation, i.e., inflammation found on both sides of the brain. Bilateral inflammation is considered worse than unilateral inflammation because in unilateral inflammation, one side of the brain can sometimes compensate for the poor functioning of the other side.
The Insula!
Inflammation in the insula alone could have been enough to cause ME/CFS.
Younger said we could have stopped right at the insula! The insula is so significant that inflammation in it alone could cause the ME/CFS symptoms. Highlighting the insula’s impact on the human experience, Younger said the insula is responsible for sensory information, bodily feelings, and most importantly, “how your emotions and your sensations and your understanding of yourself are integrated in how you ultimately feel”. The insula is responsible for making the assessment, “are you feeling well?”, to your consciousness.
Plus, because the insula also processes autonomic nervous system (ANS) signals and regulates the ANS, an inflamed insula could help produce the dysautonomia so common in these diseases. The insula is considered the “bridge” between internal bodily states and “adaptive physiological responses”; i.e., autonomic nervous system activity.
In ME/CFS, both physical and mental stressors (exertion) have been shown to increase activation of the insula, and produce an increased sensitivity to threats, increased vigilance, impaired attention, brain fog, unrefreshing sleep, and emotional distress.
The insula has been better studied in fibromyalgia, where it has been called a “central hub” in the disease. Increased default mode network activity, autonomic dysregulation, increased pain, brain fog, reduced attention, and increased
An Interoception Interlude
The idea that problems with interoception play a role in ME/CFS is not new. In 2008, in “An extended concept of altered self: chronic fatigue and post-infection syndromes“, James Jones of the CDC proposed that post-infectious fatigue states were primarily diseases of interoception.
Jones proposed that the brains of people with post-infectious illnesses were stuck in a chronic and maladaptive state of “illness behavior”. The pathogen was gone, but the brain was acting as if it was still present.
Jones’s hypothesis – that the body was essentially well but the brain was not – was controversial (and perhaps not surprisingly, he was the sole author of the paper). Jones did not, however, assert that the body was unaffected, and cited a range of biological findings (glucose and lipid metabolism, neuroendocrine issues, brain imaging findings (including the insula and thalamus) which he believed were caused by the brain’s maladaptation.
Jones’s interoception hypothesis never caught on, and the research community mostly dropped it.
Precuneus
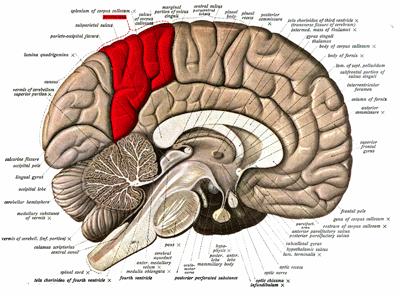
Precuneus. (Image from Dr. Johannes Sobotta, Wikimedia Commons.)
The precuneus’s close integration with the insula, and the notorious default mode network, would likely result in difficulty in focusing one’s attention and increased feelings of fatigue, pain, and unpleasant body sensations. If it seems you’re never free from negative body sensations and have trouble getting beyond them and concentrating on other things, dysregulation of the insula, precuneus, and default mode network could be the reason why.
Parahippocampus
Younger explained that bilateral inflammation of the parahippocampus has been shown in other diseases to be associated with fatigue. We see a theme emerging: like the precuneus and the insula, the parahippocampus is associated with the default mode network and interception. The parahippocampus also interacts with the limbic system, which could be amplifying sensations of fatigue, and producing anxiety and other unwelcome mental states.
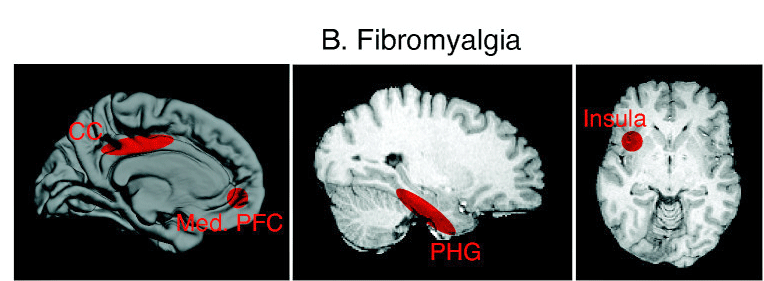
Structural changes shown in parahippocampus, insula and other brain regions in fibromyalgia. (Borsook D, Moulton EA, Schmidt KF, Becerra LR. (Image from Borsook D, Moulton EA, Schmidt KF, Becerra LR. Creative Commons, Wikimedia)
Medial orbitofrontal cortex
The fun continues with the medial orbitofrontal cortex – yet another brain region associated with the insula. Younger found “a lot of microglial inflammation” on both sides of the medial orbitofrontal cortex, which is also associated with the basal ganglia – a brain region that has been linked to ME/CFS in the past.
Inflammation in the medial orbitofrontal cortex finds a new way to keep ME/CFS patients isolated by reducing the sensation of “reward” – a key motivating factor – and by increasing “effort”.
Younger noted that when you have a fever and can’t get out of bed, activated microglia in the medial orbitofrontal cortex are one reason why.
Other Areas of Bilateral Inflammation
Younger said “lots of the other regions” such as the temporal lobe (headaches, sensory and auditory disturbances, mood swings) feature bilateral inflammation in ME/CFS, but he would talk about those some other time.
Unilateral (One-Sided inflammation)
More hits come with inflammation in the seat of fear and anxiety – the amygdala. (The amygdala had to be in there, didn’t it?) Cognition and attention take a hit with an inflamed right medial frontal gyrus. For its part, a tweaked posterior cingulate activates the default mode network – the center of rumination – which is basically kryptonite to getting things done.
Moving on, inflammation in the left hippocampus impacts learning and memory, but then we come to the left thalamus, which Younger called a “critically important” brain region that relays all sensory information that flows through the brain. Problems in the thalamus alone, he said, could explain ME/CFS.
Inflammation, Here, There, and Seemingly Everywhere
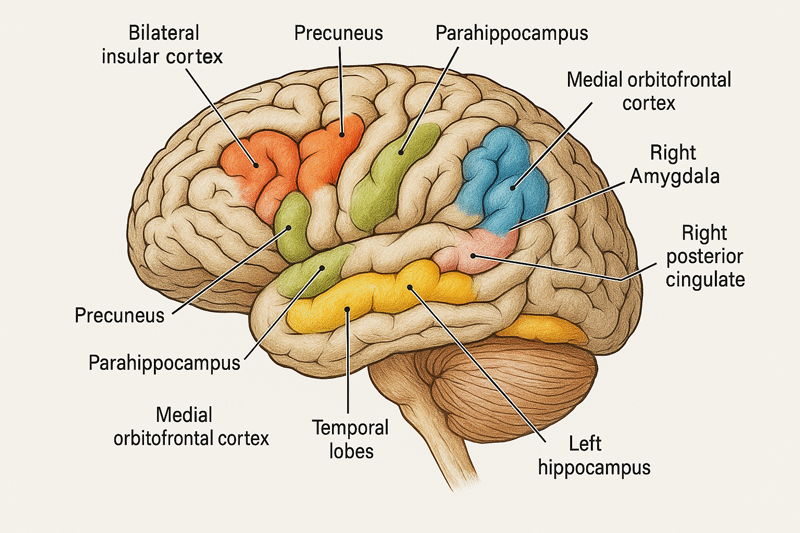
AI Perplexity image of the inflamed ME/CFS brain found by Jarred Younger.
So there you have it – neuroinflammation, Younger said, in the front, back, and in-between areas of the ME/CFS brain. At the beginning of his talk, Younger said the inflammation in the insula alone could be responsible for ME/CFS – and then added on about ten other brain regions.
That seems a little daunting! Then again, this disease was never going to be explained by something subtle. It was always going to get pretty ugly at some point – and the sooner the better. The fact that Younger found so much suggests he’s really onto something.
If Younger believed neuroinflammation was a key to ME/CFS/FM ten years ago, he’s pretty darn sure now. He believes there is now enough neuroimaging data for us to know that neuroinflammation is what we need to be targeting in ME/CFS, stating:
“We have to find or develop treatments that reduce brain inflammation if we’re going to improve ME/CFS.”

Younger started off the video with a big smile. He’s excited about what’s happening with this disease, and promised more is to come.
Younger said he hoped to have more “exciting news” on the clinical trials front soon. Also, he said there is so much going on right now that it’s hard for him to decide what to talk about each week (:)). Plus, there are a lot of “good things” on his plate right now. It’s all good news and all the more reason to stay tuned.
(Talk about a full plate. Besides the brain inflammation findings, Younger is working on his “brain invasion” study, a big “Good Day/Bad Day” study, the tFUS ultrasound device, a Gulf War Illness clinical trial, and who knows what else.)
More PET Findings Coming?
It sounded like Younger will be writing up his findings fairly soon, and we may also be in for some more PET findings. Years ago, Watanabe was reportedly in the midst of a large validation study and did publish a neuroinflammation paper in Japanese in 2018, but we’ve been waiting for the study results for so long that we can probably give up on that now.
Three years ago, Michelle Jones at Stanford was in the second year of the 2-year NIH-funded study PET scan study of the entire body. Preliminary data show increased microglial activation/neuroinflammation in “multiple brain regions of severe ME/CFS patients compared to healthy controls.” The study was due to end in 2022. Hopefully, the results will be published soon.
The Perfect Post-Infectious Syndrome Brain?

The brain Younger described seems designed to keep one from participating in life.
Has Younger just uncovered the perfect post-infectious syndrome brain? When we get an infection, the brain – not the pathogen, but the brain – in a process known as “sickness behavior”, produces symptoms, feelings, and emotions that are designed to stop us from spreading the infection, and conserve our energy so that the immune system can kill the pathogen.
This period of “sickness behavior” is supposed to end when the pathogen is vanquished, but the idea that it may have become entrenched in postinfectious diseases like ME/CFS dates back decades. The ME/CFS brain that Younger just described seems like it was designed to ensure that sickness behavior’s prime directive is carried out: the person is kept from participating in life and passing on an infection.
What kind of brain would produce that? Perhaps the brain the Jarred Younger’s new PET scan revealed.
A brain that ramps up symptoms – most especially fatigue – and body sensations to keep one from exerting oneself – and to keep one’s attention focused inwards rather than outwards. A brain designed to increase effort, blunt the “rewards” that typically move us, and reduce motivation.
A hypervigilant, anxious, and unsure brain that amplifies danger and fear and, when we step over the line, whacks us with more symptoms. A cognitively impaired brain with a short attention span keeps us from engaging in the tasks we used to engage in. A brain that impairs restful sleep keeps us in a fatigued state.
This is the type of brain that Younger found.
Two AI Engine Takes
Just for the fun of it – and noting that AI isn’t the last word in anything medical – I asked AI Perplexity and ChatGPT, without mentioning ME/CFS or long COVID.
“What symptoms and kinds of illness would a brain with bilateral inflammation of the insular cortex, precuneus, parahippocampus, medial orbitofrontal cortex and temporal lobe and unilateral inflammation in the right amygdala, right posterior cingulate, left thalamus, left hippocampus cause?”
Interestingly, AI Perplexity said it could produce a “profound sickness behavior phenotype”, encompassing global cognitive impairment, abnormal pain experience, disrupted motivation and mood, altered social behavior, etc.
ChatGPT said inflammation in these regions would likely cause “a complex constellation of cognitive, emotional, autonomic, sensory, and behavioral symptoms”. It mentioned brain fog, heart rate and blood pressure instability, problems with short-term memory, lack of pleasure, reduced reward, anxiety, sensory abnormalities, pain, fatigue, sleep problems.
Its top three clinical candidates were autoimmune encephalitis, chronic fatigue syndrome / ME (inflammatory subtype), and long COVID neuroinflammation.
A Brain Inflammation – Energy Production Link?
Since neither ChatGPT nor AI Perplexity mentioned anything regarding energy production, and at some point, the energy production problems in the immune and muscle cells need to be linked to the brain findings, I asked both engines, again, without mentioning ME/CFS or long COVID.
“Could inflammation in these brain regions impair energy production in immune or muscle cells in the body, and, if so, how?”
The answer from both Perplexity AI and ChatGPT was an encouraging “yes”.
How neuroinflammation could translate into problems with energy production in the muscles and immune system. (from ChatGPT)
Perplexity AI: Inflammation in the specific brain regions you listed doesn’t just stay confined to the brain. It actively disrupts central metabolic control, leading to systemic changes in hormone levels, immune cell metabolism, and muscle energy production. The result is impaired energy output, increased fatigue, and reduced physical and cognitive functioning. This mechanism is well documented in conditions like chronic fatigue syndrome (ME/CFS), long COVID, neurodegenerative diseases, and autoimmune illnesses involving these brain areas.
Among other things, AI Perplexity asserted that inflammation in two brain regions – the hypothalamus and insula – involved in controlling the body’s energy balance and metabolic stress responses, could lead to metabolic dysfunction. Plus, neuroinflammation in the brain could increase systemic inflammation, induce insulin resistance, and increase mitochondrial dysfunction.
ChatGPT stated: “Yes”. Through a combination of autonomic dysfunction, neuroimmune signaling, HPA axis disruption, and altered interoception/metabolism, “inflammation in the specific brain regions you’ve listed could plausibly impair energy production in peripheral cells (e.g., immune and muscle cells). These mechanisms are highly consistent with energy metabolism abnormalities observed in conditions like ME/CFS, Long COVID, and post-viral syndromes.
ChatGPT stated that sympathetic nervous system overactivity could ultimately impair mitochondrial function, fatty acid metabolism, and glucose uptake, and reduce ATP production. Neuroinflammation could also cause immune cells in the body to shift to a pro-inflammatory, energy-inefficient metabolic state.
Causes
We don’t know what is causing the microglial activation/neuroinflammation that Younger found. Probably any number of possibilities are present. Note, though, that because many of the inflamed areas of the ME/CFS brain (hippocampus, prefrontal cortex (PFC), anterior cingulate cortex, amygdala, striatum, insular cortex, orbitofrontal cortex, parahippocampal gyrus, thalamus) seem to be particularly sensitive to inflammation coming from the body, inflammation is a prime suspect.
Treatments
Younger didn’t dwell on specific treatments, but many treatments could potentially reduce neuroinflammation. They range from immune affecting drugs (LDN, IVIG, monoclonal antibodies, etanercept, PPAR Agonists, oxytocin, Donepezil, Abilify, some antidepressants) and supplements (PEA, curcumin, resveratrol, etc.) to things like deep brain stimulation, vagus nerve stimulation, autonomic nervous system affecting drugs (propanolol, etc.), and newer options like suramin, rapamycin, ketamine, as well as brain retraining (eg. “Amygdala and Insula Retraining“) to tamp down the stress response, reduce vigilance, feelings of danger, etc., could potentially help.
Younger is particularly interested in a new brain stimulation technology called tFUS, which can reach into the deeper brain regions that are inflamed in ME/CFS.
Younger noted that better treatments are needed. Hopefully, Younger’s new study will focus more attention on finding ways to reduce neuroinflammation.
Support Health Rising! Keep the Information Flowing 🙂
Health Rising is not a 501 (c3) non-profit.






Do we know what the PET scan is actually measuring as Microglia activation? As I understand it they use a radioisotope that attaches to certain receptors and that is what they measure. But the issue has always been with Microglia that the 18 kDa (TSPO) receptor doesn’t determine which activation state microglia are in, and it matters if we are to understand if its pathological or not. Clearly Jarred Younger has something showing a difference but I think we need a step of detail in what is being measured as activation. What the tracer is and how it determines activation state is going to be the critical lynchpin of the study.
He mentions the name of the tracer in the video but I couldn’t catch it. All I know is that he said its a more accurate tracer than was used in the former studies. Looking forward to the paper 🙂
From what I heard 18F-DPA-714 is the tracer. Per Wiki that is specific for TSPO as you noted. I don’t know all the details of this, I think as yet it is not validated for neuroinflammation and is still an emerging/investigational radiotracer for it, though lots of studies looking at it. https://www.mdpi.com/1420-3049/29/17/4212
@Paul Keeble If you listen carefully that is the whole point of his efforts. This second generation is meant precisely to solve that issue to prove there is neuroinflammation. Later comes establishing targets.
Interesting. I’m looking forward to his scientific publication. This is potentially the best explanation for ME. Everything he says makes sense. I hope that the second-generation radioactive tracer can conclusively demonstrate this inflammation so that more researchers around the world can verify these findings. Although I fear it will take many years before we get there, even though the cause of our symptoms may have been discovered. The next question is: what causes this inflammatory response of the microglia? I imagine that the reduced blood flow in the brain, discovered by researchers like Visser and van Campen, could be the cause of the activated microglia cells.
Reduced blood flows and problems with “neurovascular coupling” aka David Systrom, certainly must play a role.
If cardiovascular dysregulation and one of the main consequences – reduced blodflow to the brain -is a key cause (as many leading scientists believe – Syström & Co, the Scheibenbogen team, Klaus Wirth, the Norwegian team /Fluge, Mella etc) – then can you really cure this by drugs focused on neuroinflammation?
The dysregulated blodflow and reduced oxygen to cells/brain, muscles – isnt it likely to continue to cause problems in an endless cycle? Maybe you can temporary reduce “sickness feeling etc” in the brain but it will never cure anything as it doesnt target the root?
What does this mean for ME/CFS theories that claim that neuroinflammation (while implicated) doesn’t play that big a role in the core pathology? Does it contradict them?
Younger did not show – at least in this part of the study – that inflammation in these regions is associated with increased symptoms. That would probably convince some people.
One way to prove that would be to get someone better and see if the neuroinflammation resolved. Younger has found evidence of neuroinflammation using thermography, spectroscopy and PET – is confident it’s there and plays a core role. He may believe that based on what happens in diseases which have neuroinflammation.
For me, I like how the brain regions seem to fit “sickness behavior” and seem designed to keep a person down and out.
What ME/CFS theories do you know?
As far as I know there are only two: HHV-6b (or other herpes viruses?) reactivation and autoimmunity.
I think that the autoimmunity folks aren’t that interested in the brain because they are mainly experts on the immune system outside the brain. However, there is a team from Japan that had a speaker at last may’s 25 ME/CFS Berlin Charité conference. It’s interesting and I can recommend it. They seem very interested in brain inflammation and think it’s a very important part of ME/CFS.
https://events.mecfs-research.org/en/events/conference_2025/videos/takashi-yamamura-gpcr-autoantibodies-brain-alterations
Autoimmunity is still too complicated for my damaged brain to get a handle on. Therefore I can’t explain more. : )
The herpes and more specifically the HHV-6b case on the other hand is a non-brainer. I think that almost any herpes virus is at least able to produce one type of brain inflammation, and there are some who are known to cause different “benign” or highly acute and potentially lethal brain inflammation. For example HHV-1 that causes a severe type of brain inflammation that was already described in the 19th century and a “moderate” type, called Mollaret meningitis that was only understood for the first time in the 1940ies.
It could thus well be that the “moderate” form of HHV-6b reactivation was ME/CFS. HHV-6b was only found out about in the 1980ies.
People often shrug off the HHV-6b reactivation hypothesis because they think it is a too simple answer to what they see as a “complex” illness. However, from a historical perspective nothing would be off if we’d find out that HHV-6b causes ME/CFS 45 years after it was detected.
The reason why we don’t know whether ME/CFS is sub-acute HHV-6b reactivation is that nobody has done the research to define what role HHV-6b plays in ME/CFS.
Thanks Cort, this is an interesting read. I’m from the UK and took part in the DecodeME study, the initial findings of which were released this week. I’ve copied and pasted sections of the report to save energy.
“DecodeME looked at the DNA of 15,579 people with an ME/CFS diagnosis who met the study’s tight research criteria and compared it with the DNA of nearly 260,000 controls without ME/CFS. All had European ancestry, which makes the results more robust. A later analysis will look at people of all ancestries.
The initial DNA analysis is now complete, and we have made some exciting discoveries. Our results show that people with an ME/CFS diagnosis have significant genetic differences compared to the general population. These findings confirm that genes contribute to someone’s chances of developing ME/CFS. Eight genetic signals have been identified that are much more common in people with ME/CFS than the general population. The signals discovered are involved in the immune and the nervous systems, indicating immunological and neurological causes to this poorly understood disease.”
Particularly interesting with regard to the work of Jarred Younger is the finding “Another gene on the plot, SUDS3, produces a protein that dampens down the inflammatory response of the brain’s main immune cells, called microglia. So people with ME/CFS could have a weaker microglial inflammatory response in the brain.”
And….
“DecodeME used another analysis method called MAGMA to link ME/CFS genetic signals to the nervous system. MAGMA first identified a set of 13 genes associated with ME/CFS signals. Then, using existing data on gene activity, it found that this set of genes was more active than expected in 13 types of tissue in the human body (out of a possible 54), and all 13 tissues were brain regions.
Could we be getting closer?
Full report on DecodeME website.https://www.decodeme.org.uk/x-marks-the-spot/
Thanks for all you do Cort.
“The signals discovered are involved in the immune and the nervous systems, indicating immunological and neurological causes to this poorly understood disease.”
“using existing data on gene activity, it found that this set of genes was more active than expected in 13 types of tissue in the human body (out of a possible 54), and all 13 tissues were brain regions” (!!!!)
““Another gene on the plot, SUDS3, produces a protein that dampens down the inflammatory response of the brain’s main immune cells, called microglia. So people with ME/CFS could have a weaker microglial inflammatory response in the brain.”
A WEAKER inflammatory microglial response! Interesting…the plot thickens 🙂
I am very excited by this report. The very first symptom I had was a feeling of ringing and brain inflammation and it has continued to this day, only now I have added a feeling of coldness in different parts of my face. This usually occurs in the afternoon, around 3 p.m., which is the time all infections and viruses are at their worst.
Studies are always having contradictory results, often because of using slightly different protocols or the selection of patients and controls.
I have always believed that for any hypothesis, three studies should be funded using the same protocols. That way, when they were finished we would know something definitive.
Re. the genetic aspects of ME/CFS. All illnesses have a genetic component. The former head of the NIEHS used to say…”genetics loads the gun and environment pulls the trigger.” Environment can be anything from viruses to toxic contaminants.
That said, there are so many people with ME/CFS that the genetic vulnerability must be widespread.
Finally, one again we are looking a symptoms and possible treatments based on those symptoms without looking for the cause. “Cause defines the best treatment.”
The 3 things causing it are 1 closed calcium ion channels, this happens as a reaction from viral or stress. 2 lack of growth hormone, from appropriate healing pathways due to lack of activity. 3 viruses etc attack gligal cells.
The answer LOW DOSE NALTREXONE used unconventionally, it corrects all these things, I used it for 3 years plus then did Primal Trust, brain retraining, vagus and somatic, to bounce onto my natural growth hormone, high vibe from the ldn, im now ldn free and completely recovered!
I’m happy you are recovered but LDN doesn’t help everyone.
Thanks, i worked hard for it!
That’s because you’re have to understand the science behind it and experiment with It, I used approx 5mls every 3 to 4 hrs over 3 years. I also had a radical diet change,no sugar,,I eat butter instead, this fuels the gut cells with butyrate,I eat 6 foods brown rice and 2 veges they produce butyrate too,plus high grade chicken breast and organic eggs, Humans eat so much crap, it’s not a normal diet for them. PrimalTrust was key too! All the best
Congratulations, Vanessa, on your recovery! Are you aware of any others who have followed the exact same path/program back to health, or has this exact path/program been otherwise established by clinicians or researchers? Not just talking about LDN use, but the way you used it, along with adjunct therapies you mention.
Thanks alot Scot.To my knowledge im the first person to do it this way, i was desperate i couldn’t even walk 5 steps without nearly dieing, I noticed that the ldn only lasted for about 3 hrs, and symptoms started again, I did alot of online research, finding out that’s how long it kept the calcium ion channels opened then the endorphin receptors became unblocked again, I’d say the ebv was still strong in me, so I just kept dosing, I had had alot of stress in my life too, anyways I started to be able to do alot more, I had it with caffiene as it can be a bit of a downer at first, I eventually gave up the caffiene ,it was a unhealthy option of coke a cola, as I’ve had food allergies all my life and couldn’t have coffee etc, I stayed on the ldn, it was still working but without the caffiene ,it all felt like I was having too much of it, I truly feel like I was being led alot of the time, well I do pray to be shown, I was eating way too many cashew nuts and had a oxalate melt down, I flicked them and my body also decided to purge, I started looking into primal trust, you can find them on fb, and just before that found out that it is the the growth hormone receptors that are blocked to open the open the calcium ion channels, when the receptors are released it causes them to make these endorphins 100 times more, it can be quite pleasurable, it’s the hormone the body makes from sustained exercise, so then I read what I just said on Primal Trust, I had issues with my mental health from life and long term illness, by doing the course and at a guess I’d had enough of ldn to calm gligal cells down and stop the evb from replicating, I chose to stop the ldn as I felt it too depressing in large doses without the caffiene whilst I was learning a myriad of brain retraining, vagus nerve and somatic techniques and boom i move into my own growth hormone upgrade, and im still in Awe ,but very Thankful, I def need to keep stress at a minimum and do something good and athletic daily, but that’s a dream come true for me. Hope that helps. If you want my abstract I wrote on it intially for the ME cfs international Australian conference, send me a email
vgray1221@gmail.com
Thanks Vanessa for all this extra background information.
So you and some others were/are, in fact, taking normal or high dose Naltrexone! (not LDN) I’m interested to read your abstract.
How long has your journey with ME/CFS been?
The reason I’m asking is that I’ve been in it for over 3.5 decades (EBV trigger, but I’ve also tested positive to CMV, Bartonella and some kind of enterovirus), done a lot of hard work and tried just about everything, including LDN and more recently almost a year of brain retraining with Re-Origin. I’ve also spent a lot of time reading and listening to recovery stories through various channels. I also thought I was mostly recovered by my mid-thirties, but had a massive relapse (apparently viral based on the “razor blades” in my throat), due to pushing myself physically, and never bounced back like after I first went down in my twenties.
What I’ve noticed is that there seems to be a wide variety of modalities that people attribute to getting better, for the portion that do make full or near full recoveries. There are those who use anti-microbials, those who change their diets, those who figure out how to resolve past traumas and everything in between. Dan Neuffer of ANS Rewire observes similar, and says brain retraining is one thing that comes up frequently in recovery stories, but notes those who recover usually do one or two other things in parallel.
I’m wondering if the common thread in the successful modalities used for recovery is that they all work to increase the body’s perception of safety, which then allows the body to shift out of this maladaptive ME/CFS state* and go back to normal functioning. For example, if the body is fighting some kind of active infection, then anti-microbials might be key in making the body feel safe again. Equally, if someone had a lot of unresolved traumas, then resolving those, or using a lot of somatic therapies might help body feel safe again. Likewise, if someone had chronic stress from a brutal corporate job, then changing work and becoming a yoga instructor might help the body feel safe again. If this is true, then what’s important is not just apparent safety or conscious safety, but the real felt sense of safety in the body. And for some, that may be very difficult to find – it can be like winning a lottery!
I think your advice to follow the course that seems natural, whether that’s the lead of one’s god or gut intuition, seems like a sensible way to navigate through this and win the lottery. And it’s good to remember that luck might be involved too, and that simply working hard and doing what worked for someone else doesn’t guarantee success.
Thanks again for sharing and for offering your abstract 🙂
* Refer to Dr. Anthony Komaroff’s latest review here:
https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00332-5
Hi Scot ,if you check Google you will find reports of people using ldn multiple times a day and doing well on it, a lass from USA contacted me after I presented my info all over fb, she chews on a 50mg tab of naltrexone all day to keep her going, not ideal, I used to dilute mine and take measured doses, sometimes just a once a day dose works for some, it didn’t for me,it’s a steeping stone, a healing method, im glad to be off it now, I use my tools from Primal Trust to stay strong and stable.
I read through the Open Access report you suggested. Even for someone like me with a non-science background, I found the information useful to anyone trying to understand the complexities of various post infection syndromes, although the research in this post mostly focuses on Long Covid and ME/CFS. The article also pointed to the variability of causes of these post infectious disorders and pointed to possibilities for future research. It also accounted for the variability in treatments as well. Some people may find LDN helpful, and others cannot tolerate it. Some respond to certain drugs, and others find these same treatments ineffective. The article also mentioned that some of the viral catalysts are most difficult to entirely eradicate. With 204 research citations, this compilation of both issues and current treatments may be able to steer researchers in a positive direction. It also explains to some degree the frustrations that patients experience after years of looking for help from the medical community and finding so few effective treatments available.
Hi Vanessa ! As I understand 5mls of LDN is a liquid form. What would it be equivalent to if it’s pills Every 3 – 4 hours sounds a lot . I was trying 3 time to take LDN 0,5 mg ones a day , but had to stopped it because of side effects . Perhaps your approach is better. It looks like you’ve been more successful. If you can explain how you’ve done it , I would be very grateful . I’m very desperate, as my condition is very severe. Thank you in advance. Sofia
I’d like to ask you two questions: 1) Did you have any physiological testing like two-day CPET to determine your anaerobic threshold, and 2) were you ever tested for total blood volume or red blood cell mass via radioactive tracers?
Staci Stevens and Mark Van Ness did my CPET testing many years ago and found my anaerobic threshold at 97 bpm the first day and 93 bpm the second. Keeping my HR under 100 when trying to do even simple gardening or going for a walk is a major challenge. But as long as I do this, I don’t have PEM the next day and can function fairly normally while sitting at my desk.
This goes back to Dr. Nancy Klimas’s testing with EPO. She discovered that many ME/CFS patients have the problems I do which stem from not having enough blood volume and red blood cell mass to properly oxygenate the body during physical activity. We have the motivation to push ourselves, but end up crashing and burning the next day and the day after with PEM.
UCSF Nuclear Medicine did my radioactive tracer blood volume test twice, and both my total volume and red blood cell mass were significantly below normal. I also continue to deal with barely normal reticulocyte counts while having normal levels of erythropoietin. My bone marrow simply doesn’t make enough blood to meet my physical needs. Some researchers have conducted bone biopsy research and found insanely high levels of herpes viruses in the marrow, which is believed to be the cause of reduced blood cell production. But giving EPO to patients like me doesn’t seem to work for the long haul, based on Dr. Klimas’s findings. I believe her theory about chronic virus reactivation is spot on, at least for people like me.
I’m not going to discount your success, or poo-poo Younger’s findings. I just feel that some ME/CFS patients like me are dealing with a form of physical disability that doesn’t impair our emotional behavior and motivation to do stuff. We wake up and get up, ready to take on the day, but get slammed down HARD if we stay in an anaerobic state for too long. Perhaps that’s what driving the brain inflammation as it does other parts of the body?
I’d really like to see both of these camps work together and determine what someone’s total blood volume/red blood cell mass are and what their anaerobic threshold is and tightly control for it before scanning the brain. Just getting up and out to even participate in this research might be pushing these patients too far beyond their limit. Seems to me these are factors that should be known ahead of time.
Vanessa…do you or did you have any spine issues like bulged discs,alignment issues?
Hey Roonie,no not at all
Have you contacted the omf Vanessa? I’m sure they could use that kind of input
Hi Oliver, I’ve commented on thier fb stuff, I’ve contacted alot of different important people over 3 years and no one seems to care, Dr Klimas was at the cfs conference where i presented my abstract and just snobbed me, I’ve more or less just let it go as it frustrates me, Griffith university and doctors know all about but still don’t move on it,they were the ones that made the calcium ion channel discovery, if you have a contact, I’ll give it go, Thanks Vanessa
Hi Vanessa. I only know about the omf s usual willingness to engage.
Nancy klimas, she’s been in the game so ling bit seems to have generated so little. They should know we are potentially as expert as they are in our own way.
I’ll give your ideas a whirl myself tho.
I’ve noticed that I respond well to brown rice and butter myself.
Thank you for mentioning it
A few years ago when viewed diagram of Vegas nerve,observed gut brain connection,altho function not known at time . Used alpha stim,developed by inventor of ECG. Upon first use became wonder women for approx 4 years until had RSV. Currently researching again because CFS is severe following covid and flu vaccines to protect loved one undergoing cancer treatment.
Hi Joan, I do alot of self vagus work, def helps alot, alot of eye exercises excises, humming, so much you can do, Primal Trust teach alot, and I’ve even seen a vagus nerve app ,all the best
Ahh, Vanessa, we meet again! I am the person who called you out in the global ME/CFS support chats on Facebook, and I’m going to call you out here as well.
To claim that you know the singular “cause” and “cure” for any unsolved illness is both unethical and ridiculously egotistical, assuming that you actually believe what you’re saying (selling?).
To target chronically ill and disabled people who are desperate for a cure is dispicable,imo. After being booted from those chats for giving people medical advice when you are not a medical professional, and for directing people to privately message you for “more information” (or to sell them your “protocol” and direct them to sites and programs that you profit from?); I see you have moved to the comments section here.
I hope you will find that the readers of this blog are primarily better informed than you and most will not fall for your “recovery story” and weak hypotheses. All one has to do is peruse this blog for a few minutes to find that science knows A LOT more about the pathophysiology of ME/CFS than your oversimplification above.
Do you really believe that you know better than the myriad of accomplished researchers and clinicians that have devoted their lives to studying this disease? I can only conclude that you are either delusional or you’re a predator, the modern equivalent of a snake oil peddler.
Do you make money selling the “protocol” that you were offering on your “cure” in the Facebook groups? Are you compensated somehow for referring people to a specific source of LDN, or to the Primal Trust program subscriptions?
I hope not. If not, though.. if everything you’re saying is true, do you really believe that what worked for you will work for everyone? That your extremely myopic, naive, and incomplete take on ME/CFS is the definitive view on the disease… that you’ve somehow succeeded where all these research teams and multi-million dollar studies have failed? Seriously?
I know I’m speaking harshly here, and I apologize to all other readers for my tone, but I despise charlatans who target the chronically ill. Vanessa has been directly engaged by many people, sharing information, cautions, and references. She does not listen, she only argues and continues to copy/paste the exact same “story” and giving unsound medical advice to anyone who will take it. She has written up her own “protocol” based off of this and promotes it everywhere that she can.
As others have said, LDN doesn’t work for everyone. As I’ve explained before, Vanessa, even at lower doses it can be harmful for some people.
It is also unethical to suggest it to people living with more severe forms of chronic pain (or really to anyone!) without giving full informed consent about the severe trauma that can result should a person need emergency pain relief and be unable to get it due to the actions of the drug in the body.
In addition, suggesing that people with ME/CFS use caffeine or any kind of stimulant is also unethical, without also addressing the harms that use of stimulants can bring about in the longterm progression of the illness.
As someone above stated, many of us who’ve lived with this disease for 3+ decades have also thought we were “cured” at one point or another, as this illness is relapse-remitting for many. Overdoing it on a good day or a good year (with stimulants or otherwise) is a recipe for “the push-crash cycle” and subsequent crashes lowering the baseline (or functional capacity) … ie. overall disease progression and severity.
I have attempted to discuss all of this with you before, as have many others, yet you continue to push the same negligent narrative seemingly anywhere you can find where you might have access to people with ME/CFS.
I will continue to confront you everywhere I find you peddling your “protocol/recovery story”, as I truly believe that you are causing harm, regardless of whether you’re a profiteer or just an egocentric wishful thinker.
Please listen to the feedback that you get everywhere you go, and STOP POSTING HARMFUL INFORMATION.
Hey Spider, nope not made a cent thanks, was never my intention ever, its all from my heart to help. All the best to you! Have you tried it?
Just returned from a Awesome bike ride, I guess ill never know if it was my protocol or Primal Trust, combination I say, but Thankfully I did it!
Hey Spider, nope not made a cent thanks, was never my intention ever, its all from my heart to help. All the best to you! Have you tried it?
Just returned from a Awesome bike ride, I guess ill never know if it was my protocol or Primal Trust, combination I say, but Thankfully I did it!
Apparently you have been the only one that’s concerned.
And lastly, I was asked to leave one fb group, i left 2 others due to close mindedness and fear.
Hi Spider,not sure where you’ll find it, but it greatly helped a nurse here, and gave her a much better life. Also your attack is very unwarranted, you might find some help at Primal Trust for all that anger. Take care!
Needless to say I will not be replying to anymore of yr comments.
Thank you Cort and Jarred Younger. Exciting information for sure. Cort, in May you reported on how GLP1 medications are making significant symptom improvements for the ME/CFS patients of Dr. Kaufman and Dr. Ruhoy. They noted that the GLP1 medications appeared to reduce microglial activation (neuroinflammation) and enhance neuroplasticity. Given that Younger is finding that the brain is VERY inflamed indeed, it might explain why these GLP1 medications, which can cross the blood brain barrier, are making such a tremendous impact on Dr. Kaufman and Dr. Ruhoy’s patients. I have been looking for any updates that these two doctors have made since May, but I am not finding any updates or additional information on how their GLP1 treatments are going. Any chance you could reach out to them and get an update? Thank you so much for any new light you can shed on the use of GLP1 meds, now that Younger is able to prove that neuroinflamation absolutely exists in this patient population.
Hi Judi, no I’ve never had those tests, but I remember all those feelings and symptoms, I was very unwell for 10 years, had plenty of drive but no go.
Please try to read what I wrote again, low dose naltrexone opens calcium ion channels, goigle how important calciumis in yr body, stops the viral replication by soothing gligal cells, and increases growth hormone for healing ,look into those things, also check yr thoughts what you believe in and focus on you will create, All the best to you.
Hi Judi, no I’ve never had those tests, but I remember all those feelings and symptoms, I was very unwell for 10 years, had plenty of drive but no go.
Please try to read what I wrote again, low dose naltrexone opens calcium ion channels, goigle how important calciumis in yr body, stops the viral replication by soothing gligal cells, and increases growth hormone for healing ,look into those things, also check yr thoughts what you believe in and focus on you will create, i dont mean that in a mean way, Primal Trust teaches you many many emotional hacks, that eeally support your whole being. When your body is in cell danger ever, everything unchecked adds to it, so a whole system approach is wise. All the best to you.
And what has also been proven is that cfs patients have closed or compromised calcium ion channels and when they are like this that is what causes the inflammation
Nancy, thanks for asking that question. i hope there is an update from Dr, Kaufman and Dr. Ruhoy.
I heard Dr Kaufmann speak just last week about success with VERY low dose GLP-1 to reduce inflammation: “one-tenth of the dose” one might be prescribed for weight loss. This was during the panel discussion of the Aug. 14, 2025 Global Long COVID ECHO webinar series; the topic was MCAS. The recording ought to be posted in early September.
Thanks for mentioning that. Looking forward to checking the ECHO program out. 🙂
This makes sense to me. A ME/CFS specialist Susan Levine did some tests on me and found tons of inflammation. She put me on medication to help, we tested it again and my inflammation was only worse. She didn’t know why or where it came from. It is like I had lupus but I don’t. I get this heat in my body and throat that feels like steam from a dragon or something. I have MCAS which could contribute to it.
“She put me on medication to help, we tested it again and my inflammation was only worse.” How about that! Finding out what kind of inflammation and what where it is seems like its going to be crucial.
I’m kind of on eggshells waiting to see that Younger found with his brain invasion study. Given the widespread inflammation he found it would seem to make sense to this layman, anyway, that immune cells or immune mediators from the body are making their way into the brain (???)
Hi,
Many thanks for all your hard work.
Here’s a question. Does Jarred Younger address the efficacy of Hyperbaric Oxygen Treatment (HBOT) as a treatment ME/CFS and Long Covid? Jim
Not that I know of.
One part I don’t understand here. (I only read the GIST, so maybe it’s explained somewhere) but what does sickness behavior even mean? It sounds so contradictory. “There is brain inflammation and you’re acting sick when you’re not” is what it sounds like. And then the message, once again, is just quit behaving as if you’re sick and everything will be fine.
This is explained a bit better in the full text. To my understanding, “sickness behaviour” is describing all the ways that the brain gets us to rest when we are actually fighting an infection. It’s not describing any conscious processes, so definitely not another patient-blaming thing.
I understand feeling sensitive about this – for some reason the brain is treated as if it’s something we have control over. It’s kind of wild that no-one would question that we can’t control how our liver or pancreas functions, but somehow it’s assumed that we can control the much more complex organ in our skulls.
Hi, Kat, I also took exception to the description of crippling symptoms as “sickness behavior.” I read it the same way you did, as if people were looked upon as acting as if they were ill. But it turns out the entire scientific community refers to how we behave when we’re ill as sickness behavior.
It’s a technical term that goes back over 40 years!
Thank you!
This is A Big Deal! I totally agree with what Jarred Younger is saying here that ME/CFS patients’ brain is an inflamed brain. After all, this is what Encephalomyelitis means – brain inflammation.
Personally, I had experienced many different early symptoms that were brain related, eg. footdrop, one sided muscle weakness then affecting both sides, strange migratory joint pain throughout my whole body, sleep problems, extreme brain fatigue, body fatigue, sensitivity to noise, light and smell, and extreme thirst – all of which happened suddenly. That was 32 years ago, when I was 38 years old. I was bedridden for 4 years, but like many others with ME, I developed severe POTS, which is most disabling. So now I am mostly On my bed, rather than In my bed 🙂
I remember hearing about Dr Jay Goldstein, the brain expert, back in the 90’s and reading his book, Betrayal by the Brain. He explained about the brain’s dysfunction and dysregulation in ME/CFS. (He also used SPECT and PET scans). That helped me understand that this is a brain injury – or Neuroinflammation!
So now, some 30 years further on, what Jarred Younger is discovering more about our sick brains is amazing! I cannot wait to hear more from him! This is what we need more of… someone who is totally on the ball.
Thank you Jarred!
And thank you Cort for all the updated info!
I remember “Betrayal by the Brain”! It was so technical that I had a lot of trouble getting through it. I doubt I ever really did. IT would be interesting now to go back and see how much he got right. I wouldn’t be surprised if it was quite a bit.
Does no one here know that AI is designed to amplify confirmation bias? Really shocked at the excitement because AI told you something you wanted to hear.
AI is not an all seeing entity. It’s literally a data scraper, and worse, it hallucinates whatever will please you and keep you coming back for more. It’s designed to do so.
Ask it to write you a CV for example. It just makes shit up, which hopefully you’ll know because you know your own career.
Academic integrity officers are being driven crazy by student, and professional papers that appear legit, and are full of fictional references.
And yet there’s no mention of any kind of rigorous (or otherwise) examination of its results here. Come on, people.
AI is a tool like any other tool – it has its uses and its problems. Simply dismissing AI just because it has some problems is not dissimilar than depending on it for everything. For me, I thought it was interesting that after shoveling the brain inflammation findings into it it came up with sickness behavior without being prompted. Ditto, the fact that without prompting, it associated the brain-energy-immune-muscle issue with ME/CFS.
It’s clearly really good at pattern recognition; the question, of course, is are the patterns accurate? So, I few it as more of a kind of game to see what it comes up.
Look, sure, and hats off to your work here which gives hope to many ME sufferers. I’m not simply dismissing AI – it’s a topic of discussion in my circles of both artists and researchers. I guess as a journalist of 30 years, with a PhD (and sufferer of ME/CFS), and as a health reporter amongst other things, and knowing how this stuff infects our knowledge systems… at the very least with such a game or experiment, personally I’d be asking for its sources, and checking those sources exist, and they say what AI says they’re saying… before sharing AI hallucinations.
Various AIs swiftly assess your areas of interest and as I said, confirmation bias is a serious problem.
I have friend in academic integrity at a top sandstone university, and it’s a nightmare – students and academics alike drawing from dubious sources because AI said so. And another who is an epidemiologist assessing meta-analyses and again, AI slop dotted all through it. That’s all well and good in the arts, but when you’re in science and health it becomes a big problem.
And for yourself, when you are regarded as a serious resource, as you are, you undercut your own work playing AI games as an exercise.
Before you know it you’re participating in the production of AI ‘slop’ that then risks integration into genuine knowledge bases.
THat said, yeah, I’d be curious for a follow up checking on the sources (and properly checking that said sources exist, and the reference pages are correct!).
Once again, thank you for your fascinating work. Myself and a CFS friend are both finding, anecdotally, that mounjaro is changing our energy levels in significant ways, and we both were excited to see what you had found in that area.
Here are the sources AI Perplexity Pro used in answering the question ““What symptoms and kinds of illness would a brain with bilateral inflammation of the insular cortex, precuneus, parahippocampus, medial orbitofrontal cortex and temporal lobe and unilateral inflammation in the right amygdala, right posterior cingulate, left thalamus, left hippocampus cause?”
I hope this is readable (lol)
karger.com/ced/article/49…
Isolated Insular Stroke: Clinical Presentation – Karger Publishers
link.springer.com favicon
springer
link.springer.com/article/10.100…
Clinical presentation of strokes confined to the insula: a systematic …
.
my.clevelandclinic.org favicon
Cleveland Clinic
my.clevelandclinic.org/health/symptom…
Brain Lesions: What They Are, Causes, Symptoms & Treatment
Brain lesions are areas of injured or damaged brain tissue. These can happen for a wide range of reasons. Depending on the cause, some are treatable or even curable.
scielo.br favicon
Arquivos de Neuro-Psiquiatria
scielo.br/j/anp/a/6pQYpV…
Migraine improvement correlates with posterior cingulate cortical …
ABSTRACT Objective The main goal of this study was to correlate migraine improvement, after…
bmcpsychiatry.biomedcentral.com favicon
BioMed Central
bmcpsychiatry.biomedcentral.com/articles/10.11…
Reduced functional connectivity between bilateral precuneus and ...
ncbi.nlm.nih.gov/books/NBK532981
Frontal Lobe Syndrome – StatPearls – NCBI Bookshelf
sciencedirect.com favicon
sciencedirect.com/topics/medicin…
Medial Orbitofrontal Cortex – an overview | ScienceDirect Topics
Insights into Imaging
pmc.ncbi.nlm.nih.gov/articles/PMC48…
Bilateral temporal lobe disease: looking beyond herpes encephalitis
Nature
nature.com/articles/s4159…
Amygdala enlargement and emotional responses in (autoimmune …
Proceedings of the National Academy of Sciences of the United States of America
pmc.ncbi.nlm.nih.gov/articles/PMC43…
Asymmetrical effects of unilateral right or left amygdala damage on …
Nature reviews. Neuroscience
pmc.ncbi.nlm.nih.gov/articles/PMC26…
Pain and Emotion Interactions in Subregions of the Cingulate Gyrus
BMJ Case Reports
casereports.bmj.com/content/15/9/e…
Unilateral thalamic oedema secondary to venous sinus thrombosis
neurolrespract.biomedcentral.com favicon
BioMed Central
neurolrespract.biomedcentral.com/articles/10.11…
Isolated thalamic stroke – analysis of clinical characteristics and ...
Biological Psychiatry
pmc.ncbi.nlm.nih.gov/articles/PMC49…
Hippocampal Neuroinflammation, Functional Connectivity, and …
Depression, a condition commonly comorbid with multiple sclerosis (MS), is associated more generally with elevated inflammatory markers and hippocampal pathology. We hypothesized that neuroinflammation in the hippocampus is responsible for …
PubMed
pubmed.ncbi.nlm.nih.gov/26732827
Intrinsic functional connectivity of insular cortex and … – PubMed
ncbi.nlm.nih.gov/books/NBK570606
Insular Cortex – StatPearls – NCBI Bookshelf
sciencedirect
sciencedirect.com/science/articl…
Anterior insula morphology and vulnerability to psychopathology ...
healthline.com/health/tempora…
Temporal Arteritis: Symptoms, Treatments, and Causes – Healthline
Temporal arteritis occurs when the temporal arteries, which supply blood to the head and brain, become inflamed or damaged.
nhs.uk/conditions/temp…
Temporal arteritis – NHS
BMC Neurology
pmc.ncbi.nlm.nih.gov/articles/PMC49…
Bi-insular cortical involvement in anti-NMDA-receptor encephalitis – a case report
Journal of Neuroinflammation
pmc.ncbi.nlm.nih.gov/articles/PMC59…
Neuroinflammation is increased in the parietal cortex of atypical Alzheimer’s disease
BMC Neurology
pmc.ncbi.nlm.nih.gov/articles/PMC34…
Increased left anterior insular and inferior prefrontal activity in post-stroke mania
frontiersin.org/journals/psych…
Frontiers | Gross anatomical features of the insular cortex in affective disorders
frontiersin.org/journals/neuro…
Frontiers | The amygdala–insula–medial prefrontal cortex–lateral prefrontal cortex pathway and its disorders
pubmed.ncbi.nlm.nih.gov/34547400
Anterior insula morphology and vulnerability to psychopathology-related symptoms in response to acute inflammation – PubMed
Frontiers in Psychiatry
pmc.ncbi.nlm.nih.gov/articles/PMC11…
Gross anatomical features of the insular cortex in affective disorders
The sources AI Perplexity Pro used in answering this question “Could inflammation in these brain regions impair energy production in immune or muscle cells in the body, and, if so, how?” honestly made more sense to me.
medicine.washu.edu favicon
WashU Medicine
medicine.washu.edu/news/brain-inf…
Brain inflammation triggers muscle weakness after infections
Clinical Advisor
clinicaladvisor.com/features/brain…
Brain Inflammation May Be Reason Behind Muscle Fatigue After …
Frontiers
frontiersin.org/journals/behav…
The High Costs of Low-Grade Inflammation: Persistent Fatigue as a …
Frontiers
frontiersin.org/journals/immun…
Metabolic Control of Smoldering Neuroinflammation – Frontiers
Anna Marsh
annamarsh.co.uk/neuroinflammat…
Neuroinflammation: The Big Rocks For Brain Health – Anna Marsh
http://www.americanbrainfoundation.org
americanbrainfoundation.org/muscle-weaknes…
How Neuroinflammation Causes Muscle Weakness
Frontiers in Cellular Neuroscience
pmc.ncbi.nlm.nih.gov/articles/PMC43…
Systemic Inflammation and the Brain: Novel Roles of Genetic …
National Gaucher Foundation
gaucherdisease.org/blog/systemic-…
How Systemic Inflammation Affects Your Brain & Central Nervous …
Journal of Translational Medicine
pmc.ncbi.nlm.nih.gov/articles/PMC11…
Neuroinflammation and energy metabolism: a dual perspective on ...
Frontiers
frontiersin.org/journals/aging…
The role of neuroinflammation in neurodegenerative diseases
sciencedirect.com favicon
sciencedirect
sciencedirect.com/science/articl…
Inflammatory processes in the prefrontal cortex induced by systemic …
Brain and Neuroscience Advances
pmc.ncbi.nlm.nih.gov/articles/PMC70…
The role of the immune system in driving neuroinflammation – PMC
sciencedirect.com/science/articl…
The influence of systemic inflammation on inflammation in the brain
sciencedirect.com/science/articl…
Immunometabolism in the Brain: How Metabolism Shapes Microglial …
sciencedirect.com/science/articl…
Low-grade chronic inflammation, skeletal muscle wasting, and brain …
nature.com/articles/s4139…
Role of neuroinflammation in neurodegeneration development
Free radical biology & medicine
pmc.ncbi.nlm.nih.gov/articles/PMC50…
Energy Metabolism and Inflammation in Brain Aging and …
The Functional Neurology Center
thefnc.com/research/do-yo…
Do you have Brain Inflammation? How to know and what to do
Nature Reviews Rheumatology
go.gale.com/ps/i.do?id=GAL…
The brain and immune system prompt energy shortage in chronic inflammation and ageing
science.org/content/articl…
ScienceAdviser: Inflammation in the brain trips up muscles
SciTechDaily
scitechdaily.com/hidden-connect…
Hidden Connection Discovered: New Research Links Brain Inflammation to Muscle Fatigue
PubMed
pubmed.ncbi.nlm.nih.gov/29021568
The brain and immune system prompt energy shortage in chronic inflammation and ageing – PubMed
S
India Today
indiatoday.in/health/story/b…
Brain inflammation linked to muscle fatigue, new study finds
genengnews.com/topics/transla…
Brain Inflammation and Muscle Weakness Linked by Signaling Axis
yahoo.com/news/brain-inf…
Brain inflammation may be the reason behind muscle fatigue after infection and injury
Conditions such as long COVID-19, Alzheimer’s disease and meningitis can damage the line of communication between your brain and your muscles.
studyfinds.org/brain-circuit-…
Researchers uncover secret connection between brain and body’s response to disease
The Journal of Neuroscience
pmc.ncbi.nlm.nih.gov/articles/PMC73…
Acute Inflammation Alters Brain Energy Metabolism in Mice and Humans: Role in Suppressed Spontaneous Activity, Impaired Cognition, and Delirium
ncbi.nlm.nih.gov/search/researc…
News: Inflammation in Brain’s Immune Cells… (The Scientist) – Behind the headlines – NLM
Cort! This is impressive!
Yes, I do, Gretchen. I have a very basic understanding of AI but what I think I understood is that you mustn’t use it in the way that Cort uses it. ; )
Thanks for bringing that up!
Thanks, Cort.
It’s a subject I’ve been immersed in for years, though it’s rarely tested or acknowledged.
Yes, the brain is inflamed. But the real question is: what’s causing it — a virus, bacteria, or fungi?
Brain Inflammation: Finding Evidence for TMPRSS2, Encephalitis, and Meningitis
https://swaresearch.blogspot.com/2025/03/brain-inflammation-finding-evidence-for.html
Herpes Simplex Virus and Its Impact on the Brain: Understanding HHV-6 Encephalitis
https://swaresearch.blogspot.com/2025/05/herpes-simplex-virus-and-its-impact-on.html
Understanding the Skull-Meninges-Brain Axis and Multifocal Meningoencephalitis
https://swaresearch.blogspot.com/2024/11/understanding-skull-meninges-brain-axis.html
It is a pity that some neurologists in Germany still do not recognize the connection between Brian infection (virus, bacteria and fungi), LC and ME CFS and classify patients as hypochondriacs.
It’s good that viruses, bacteria, and fungi are mentioned here. I’ve had ME/CFS for 13 years and had incredibly severe problems with systemic candida in the early years.
So, after many attempts to combat the fungus, I took fluconazole for 8 weeks. After about 5 weeks, I woke up in the morning and all my symptoms were gone. It was as if a window had opened, and I couldn’t believe my luck at being able to feel normal again. After 2 days, this window closed, and all my symptoms returned. No one could explain this to me, and it hasn’t happened again to this day.
Perhaps a good approach!
Thank you Sieglinde
You’re welcome, and thanks for sharing.
It’s time for science to look deeper.
Symbiosis Between Viruses and Bacteria – A Fascinating Partnership in the Microbial World
https://swaresearch.blogspot.com/2025/07/symbiosis-between-viruses-and-bacteria.html
Michi,
Something similar happened to me about 10-15 years ago. I went to a dermatologist for a mole. Before even looking at my mole she looked at my face and said, “you have a generalised fungal infection”. She prescribed Fluconazole for 8 weeks too. After 3 weeks, my brain fog completely disappeared; my energy came back. I was very surprised as I was not expecting that. Unfortunately, that effect wore off after 10 days.
Infections definitely play a role in this disease.
I think this is not first study which shows neuroinflammation in ME/CFS patients.
Watanabe shows 34% of 76 ME/CFS patients have neuroinflammation especially in thalamus and brainstem .
It correlates antioxidant index and immune index in serum .
After anti microglial activation drug (ibudilast) treatment, symptoms were improved.
And it was correlated with decrease of neuroinflammation in midbrain.
It also improved in insula.
By the way I believe I have read published article a few years ago but I couldn’t find one. 🤔
The report below has different contents between in English and in Japanese. So please translate when you read.
https://www.amed.go.jp/content/000083247.pdf
I forgot to mention about the important information about this study. Marker [18f]dpa-714 was also used .What I meant is showing neuroinflammation in some ME/CFS patients using this marker was already demonstrated.
According to Wikipedia, Ibudilast is used mainly in Japan, crosses the blood–brain barrier, and suppresses glial cell activation. Have any ME/CFS patients in Japan tested the effects of Ibudilast?
Yes. Many patients were prescribed ibudilast. It’s not silver bullet but it seems to improve ME/CFS symptoms especially brainfog and headache. Some patients report it makes symptoms better entirely. Although Doctors said best treatment option are rTMS.
Yes. Many patients were prescribed ibudilast. It’s not silver bullet but it seems to improve ME/CFS symptoms especially brainfog and headache. Some patients report it makes symptoms better entirely. Although Doctors said best treatment option are rTMS.
Great summary!
Just a reminder: “neuroinflammation” is a somewhat controversial term, and here Jarred shows that in ME/CFS microglia is in an “activated” functional state compared to healthy people. There are also other types of glia partaking in and reflective of “neuroinflammation”: astrocytes (which are important for neurovascular coupling and also regulate the blood brain barrier, for instance) and oligodendrocytes (which insulate neurons and thus influence connectivity between brain centers). Unfortunately, only microglia can actually be tagged reliably, so there are still a few unread chapters of “neuroinflammation” or impaired brain function in general.
There is a lot of circumstantial evidence though that both the astrocyte compartment and oligodendrocytes play a very important role in the dysfunctional “ME/CFS” brain. We have summarized this in a review paper: https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2022.888232/full – on which you, Cort have also reported: https://www.healthrising.org/blog/2022/06/23/neuroglial-hypothesis-chronic-fatigue-syndrome/
Jarred´s work is a BIG step to a better understanding of ME/CFS. It is important to recognize that peripheral findings like muscle weakness or endothelial dysfunction can all have a “central” (brain-related) root and vice versa (what you have nicely worked out, Cort). Thank you, Cort!
I kind of held my breath when I punched in the question: could the brain be producing these problems in the body as well! It was good to see that both AI search engines believe it can, anyway.
Here’s to some the development good tracers for astrocytes and oligodendrocytes!
Cort, I think we have it established now that ME/CFS starts in the immune system.
What we know now is that there is a specific T-cell pathology in ME/CFS (that points to chronic viral reactivation)
I think that doctors like Avindra Nath claim (without any factual basis) that ME/CFS is a brain disease – in the sense that all the symptoms can be explained through a brain pathology – has to do with the fact that they are very faithful to their community who has claimed for decades that ME/CFS is a psychiatric disorder.
This claim has successfully been refuted. But these doctors think that they could not been totally wrong and so they now believe – again without any evidence – that ME/CFS then must be the cousin of the psychiatric disorders, a neurological disorder.
I am happy that Jarred Younger makes such good progress. However, I don’t think it is sound to project all your “the brain as the cause”-speculations into his findings. I find it unfair actually to see that you use his hard won and very important results about brain inflammation only to push your own totally speculative ideas.
Let’s keep our feet on the ground and stay with the established facts: Often ME/CFS is triggered after a blow to the immune system and starts from there. There is not one serious researcher in the field that is contesting that.
I find it difficult to understand how one could believe that a disease that starts in the immune system is actually a brain disease in which the brain causes the problems in the immune system – where we know in an incontestable way that the disease started.
Honestly, I don’t understand why you keep following such a wild idea.
It’s all speculation at this point Lina. Nothing the matter with speculating. I think we should speculate as much as possible.
Nath, by the way, has said that he thinks B-cell problems start everything off. A recent blog suggested that it all starts in the immune system.
Time will tell!
I disagree, Cort. There is certainly more than speculation going on at this point.
The herpes theory has been around for decades and we’re seeing now a lot more research into it, because many prospective studies have shown that herpes does play a role in ME/CFS. Now they are doing the research needed to fully understand that role.
The autoimmunity theory is difficult to judge for me because it’s such a complicated matter and I haven’t any base in understanding the immune system because there wasn’t anything tought at high school about it. Much differently from viruses.
However, if you read the summary of the new projects of Akiko Iwasaki, or researchers in C. Scheibenbogen’s team in Berlin they can describe their research question in a relatively clear and simple way, and I hope that they formulate their questions in a way that their empirical results will them them whether there is something about their ideas or not. But again, I can’t judge it.
In both fields the researchers are way further than merely speculating.
I am not sure whether you understand that speculation is an important part of science but it will not lead you to empirical results. You will get empirical results by formulating a simple and clear question that you then put to test and the outcome will either tell you that your hypothesis doesn’t hold or you will find evidence that your hypothesis seems to be supported by what’s really going on in the biological processes that you want to understand.
What you are doing in this blog is totally useless. You can’t corroborate a new idea, a hypothesis about how something might be working in nature by using AI. AI only “knows” or rather manages information on what’s already been found out.
The reason why AI is more and more used in research is because it can help with big statistical analyses and similar things. But it is always the case that the researchers have to formulate a question first and then have to design a programme that uses AI to answer it, and not because we can now simply ask AI to do research.
New insights are generated by asking simple and clear questions and put them to test. Speculation is a part of the process of finding a research question. But you have to do many other steps like reading and judging the literature and understand what’s already been found out. In the field of ME/CFS it especially means that you weigh hypotheses on their level of likeliness by understanding by how much evidence they are already supported or not.
I’ve said it before here. But I will repeat it. The most important thing for us as a patient community is to get everyone to understand that herpes is our hottest candidate and the easiest to go after so that we can as fast as possible fully understand it’s role in ME/CFS.
It is not our job to demand that researchers speculate more about hypotheses that are pretty outlandish. Just as Nath’s brain idea is. On the contrary, as a patient community we should demand that these question are given prority that have the strongest empirical support so far.
I didn’t read anything above that stated that the disease state is *caused* by neuroinflammation; I read that many *symptoms* can be explained by neuroinflammation. There’s nothing above that rules out an initial immune-related “cause.”
I think you lean a bit too far to the other side when you dismiss any possible neurological etiology and claim that viral triggers are a sole “cause” for everyone.
What we know now is that a complex mix of immune, neurological, genetic, metabolic, and other factors interact (often multi-directionally) to both create and maintain this disease state.
Initial illness trigger(s) are often viral, but not always, and the subsequent development of the illness is widely variable and very individualized.
As research continues to pull apart these different threads, the complex picture emerges. I’ll say again that it seems many of these processes are multi-directional. The body, and especially the brain, regulates itself in complex feedback loops.
It’s highly likely that an immune (and/or autoimmune) process could trigger neuroinflammation, which then triggers other multi-system cascade effects, which all feed back on themselves to further disease (and symptom) progression.
If the disease were purely immune, couldn’t that also be affected by processses in the brain that help regulate immune function?
Isn’t it also possible that immune dysfunction and/or autoimmunity could directly affect the brain, which then causes cascading effects throughout the body?
I feel like we know enough to say that it’s likely “All of the above.” … and then some. What we’re still looking for are the missing connections between all of these findings.
Arguing for one incomplete view over the other seems short sighted to me. Why wouldn’t such a complex, multi-system disease also have a complex multi-system etiology?
…
I also just want to say Thank You, Cort! .. for all that you do! What a critical service you provide to this community! I hope you know how deeply appreciated you are.
I am a stout advocate of the theory that HHV-6b reactivation is the driver of ME/CFS.
There’s already a lot of research around that suggests this could be very likely be the case. A scientist in London, Jackie Cliff, leads the research into fully determining the role of HHV-6b reactivation im ME/CFS.
The hypothesis is fully explained here:
https://www.brunel.ac.uk/research/projects/reactivation-of-herpesviruses-in-chronic-fatigue-syndrome
HHV-6b is not thought to be the trigger of ME/CFS. It’s thought that all the known triggers – EBV, COVID, vaccinations, accidents, cancer therapy, and chronic stress – damage the immune system in a way that it is not anymore capabe to control the reactivation of this latent virus.
I am one of many patients who experiences a herpes drug, aciclovir as fully effective to suppress ME/CFS flare-ups and also more severe episodes. A fact that is known among specialists and patients since decades.
Since aciclovir has good activity against HHV-6b I believe that the HHV-6b hypothesis is by far the best hypothesis that we have, and as patients we should demand that ME/CFS research makes it their priority to fully track down the role of HHV-6b as forcefully and as fast as possible.
The idea that ME/CFS is a complex disease is not a fact but is strongly tied to the idea that autoimmunity is the cause of ME/CFS. It means this: If it is the case that ME/CFS is an autoimmune illness, the autoimmune processes that cause it and the damage they bring to the immune system, the vessels, the mitochondria, the brain ect. would be complex and extremely difficult to understand.
Since there is no proof that ME/CFS is an autoimmune illness the idea that ME/CFS is a complex illness is speculative.
It is not a sign of good research to think that the research object is extremely complicated. Good science formulates simple and clear hypotheses that it then puts to an empirical test.
Therefore also from the standpoint of the theory of science herpes theory is our best shot.
I encourage you do your own research on the role of HHV-6b in ME/CFS. If you do it in an open-minded attitude you will understand that this hypothesis is in any respect and by far the most clear and convincing idea of the cause of ME/CFS.
Hello Cort, good article, fascinating and valuable research.
Have you picked up yet on the DecodeME DNA study from the UK? Excellent results, ME/CFS patients are distinguished by genetic differences that influence the immune response to infection, the nervous system and pain centres. What a result! Fits exactly with so much prior research and the symptomology. Is it too presumptive to suggest that an unlucky disease history + a genetically inherited risk for an aberrant immune response and neuro inflammation = ME/CFS?!
I think that’s a really nice scenario and how nice that findings – whether they are in the brain, the immune system or the genes – seem to be coming together Thanks for the tip, Nigel 🙂 A blog is coming up.
I don’t believe Herpes is a valid trigger because the vast majority of the world’s population has had Herpes yet does not have ME/CFS. I never had a triggering illness that I am aware of and yet I still have this disabling condition. Herpes is one theory. Based on my symptoms (headaches, foot drop, tingling, frequent urination, sleep problems, memory problems, exhaustion, malaise, etc.) I’m more inclined to believe that ME/CFS is a neurological/CNS condition. We’re not ready to put our eggs in one basket yet and quite frankly, the causes could be different for different segments of this community. I’m not convinced this is a monolithic condition.
It is now very clear that herpes infections play a role in multiple sclerosis. The problem is that in only a very few people does it do that to. The same is true for ME/CFS. They’ve also been shown to trigger ME/CFS in college students (see Lenny Jason’s paper.) Actually, it’s a great virus to produce neurological problems. On the other hand, there are likely many triggers and it’s just one of them.
Theanine, an amino acid, deactivates microglia.
I am improved greatly taking 400 mg 4x daily
Never see it mentioned.
Thanks, I’d like to try this. Is it L-Theanine? Which brand do you have positive experiences with? THank you!
My daughter’s statement when illness overtook her life in 1997: “My brain feels like it’s on fire!”
🙁 I’m so sorry. I worked so hard on this illness and feel like I’ve gotten far, considering. I’ve even helped my brain a little but really, I feel that, at least, for me, I don’t know how to really help my brain or my immune system
Great post as always.
But I must admit I am not a fan of the ChatGPT and Perplexity additions. In my experience when using these AI tools in trying to understand my own condition, they will happily generate very plausible-sounding content, but I often cannot corroborate it with any source. This includes when these tools give sources themselves: often I find that a source might even say the opposite of what the AI claimed.
In my opinion, I think these tools are great for pointing me in the right direction, but anything they say MUST be corroborated by an authorative source. If such a source cannot be found it’s overwhelmingly likely that it’s just presenting something plausible (which may be hallucinations or remixed from sources that you would immediately dismiss in the course of your own research).
Unfortunately I think using these tools in the way you are here is degrading the otherwise excellent quality of your article, and IMO also has a negative impact on its credibility. I mean no offense, it is intended to be constructive criticism.
I think we need to develop a cultural expectation of AI literacy. Imo, we should all automatically view anything we’re told by AI with a huge amount of skepticism.
AI can be viewed as a more sophisticated search engine, which, as you say, may point us in a more specific direction with our research. The next step should always be to verify every claim and every source. This is true for traditional research as well, why wouldn’t it automatically translate to the use of AI?
To me, it’s a useful tool for brainstorming hypotheses and compiling sources. It’s then my job as the researcher (or the reader) to do my due diligence (as energy allows). When I read the above, I was intrigued by the results, while also holding a healthy skepticism. It seems to me this skepticism should just become our default setting.
Fighting AI is a futile use of precious energy. It’s already here and is a trillion dollar, world changing technology. It’s only going to get bigger.
All we can do now is attempt to develop skills, social awareness, ethics, policies, and laws to direct how this tool should be used.
I personally find the use of medical AI in research very hopeful, though I am admittedly ignorant about how it differs from the AI that us common folk have free access to. If anyone here knows more about that topic, not only would I love to hear it, but I think it’s very relevant to the field we all share an interest in.
Many thanks!
This is what I tell people ME/CFS is: Inflammation of the brain. Especially when only a short answer is wanted. I like watching Jarred Younger’s videos on YouTube and felt like he was getting more close to answers than almost anyone else. Of course I forgot about him because of my brain inflammation. So excuse me while I go check out his new videos. Thanks for this article!
The brain is not a enigma, feed it the right things and it will change, create new neurotransmitters, this may also come in the form of vagus and somatic work as well as brain retraining, but if your serious about healing, you’d been mad not to do Primal Trusts extensive and comprehensive healing course,unlike what you may currently be able to even imagine. Truly has transformed my life and was a big part of my puzzle! The more you focus on this illness, the more it creates.
Primal Trust Academy and Community can be found on fb to follow for free, or join them and journey to wellness.
Is the Primal Trust program available only on Facebook? If so, be aware that Meta owns the copyright for everything you post there — it’s in the fine print that you endorsed when you signed up to Facebook. If it is only available on Facebook, why is that? If it’s as useful as you say, surely it could be promoted to a wider audience — like ANSrewire and other recovery programs.
Just google Primal Trust Academy and Community, they just advertise on fb, thier course is run the Simplero app
By the way for anyone suffering from fibromyalgia, Tonix Pharma had its new drug approved by the FDA today. The medication, a sublingual formulation of cyclobenzaprine, is the first new fibromyalgia drug in over 15 years. It is designed to address fibromyalgia-related pain, sleep disturbances, and fatigue.
Thank you Cort, as ever, for this excellent article. I really thought I’d found the answer to a question I’ve been posting on other forums. I thought that the ‘inflamed brain’ absolutely described what my son has been experiencing since 2003. But now I’m unsure. So I shall repost my query and hope among the hugely informed of your readers , someone can help us.
In 1994, at the age of twenty-one, our son, a Cambridge university graduate, was diagnosed with ME, having fallen ill while reading Social and Political Science. Recently I wonder if he suffers from a different undiagnosed illness and write to see if I can find a desperately needed answer.
Originally he suffered extreme fatigue to the point of being unable sit up or speak, classic symptoms. In 1996 I discovered a unit in the National Hospital of Neurology and Neurosurgery, London, treating people with ME and other rare conditions. In 1997 he spent ten weeks there, by the end of which he could walk, talk to other patients and begin to rebuild his life. Walking was essential, six times a day for half an hour each time.
By 2003 he was 50% recovered: driving, teaching music, studying again, working in an Oxfam bookshop, until he was struck down with glandular fever / Epstein-Barr virus, medically verified.
He immediately developed a weird indescribable sensation, deep within the skull or brain which makes him feel very ill, and which limits him to many hours lying down. He has tinnitus and chronic constant vertigo, which started at the National Hospital. Has recently developed vestibular migraine, diagnosed by Mark Kellett at Salford Royal. All ‘brain symptoms.’
He’s consulted well-known UK doctors treating ME, and several neurologists. Had antivirals, LDN (causing headaches) and every alternative medicine possible. Spent a fortune at the Breakspear Hospital, Hemel Hempstead for alternative medicine. Nothing has changed these symptoms He believes this indescribable ‘headache’ is his version of ME. But if he no longer has the classic symptoms, what else can it be?
He continues to persevere, but is more and more incapacitated, the head sensation worsens if he applies his mind to tasks. He feels constantly very ill, though he does not experience the original fatigue. I’d read here about intra cranial hypertension but he feels this is not the cause. We contacted Dr Higgins, Cambridge, but he no longer treats patients.
If anyone reading this can recognise what this is, has experienced it and can advise us or suggest medication or some process, we would be immensely grateful.
OMG – what your son has gone through…I don’t know if you remember but Lauren Hillenbrand, the author, suffered from horrific vertigo for years. At some point I remember her health improved greatly. Unfortunately, she never, to my knowledge, told the ME/CFS community how she got better. I have not heard anything of her in years.
We also have a recovery story in which the guy, who suffered from dizziness 24-7 and had a rough time getting a diagnosis, recovered using HBOT.
https://www.healthrising.org/blog/2024/12/31/hyperbaric-oxygen-therapy-long-covid-chronic-fatigue-syndrome/
Good luck with your son.
Hello Cort, Thank you so much for taking the time to reply. I’ve followed this blog for years and read your articles in ME Research. I don’t remember Lauren Hillenbrand but your suggestion about HBOT is very welcome. Thank you, thank you, Sue
Simply sending support as another mother; full of admiration for your perseverance and determination over such a long period of time.
Thank You, Kim. That is what we do, that is who we are!
Cort does this explain why patients have high ICP(intracranial pressure)? It is a much simpler test to prove inflammation and treating it can help.
It could in several ways – by disrupting the blood brain barrier, by promoting pro-inflammatory cytokines that impact the blood vessels which in turn can affect ICP, by and by altering the production of the cerebral final fluid. Interesting stuff!
My ME/CFS was started when I was attacked by three guys on the street. I had a whole can of mace sprayed into my face, in my eyes, up my nose, down my throat, my lungs. My face swelled up like a bulldog’s. Soon, the muscle cramping and a constant migraine were accompanied by intolerance of scents and chemicals. I iced my head down during the day and at night for at least a year. It was the only thing that calmed the headaches. At the time I suggested to my doctors that the mace crossed the blood brain barrier, which had let down because I was in a high stress fight or flight mode. The docs said it wasn’t possible that the blood brain barrier let down, but clearly it did. That was 35 years ago. While not being active partially addressed the pain and PEM, I’ve spiraled into all the comorbidities—POTS, etc. I feel neuroinflammation is clearly involved in my story along with genetic predisposition.
Jerrod Younger has it right. I’ve been telling people all along that I can feel when the inflammation rises in a specific spot in my brain and always grab my head. It’s my last clue to stop what I’m doing and rest or else !
I also have had narcolepsy from the beginning, and all the usual co-morbidities. I finally get one quieted down and another decides to show up. Like Sjogrens Syndrome, etc.
Also, when you speak of post-viral, I have a history of herpes simplex that started at age 16 and lasted for years. So every time a researcher comes up with something, I identify with it.
Two questions :
Is there a safe place to donate our brains for research ?
Is there a directory of physicians who believe in ME/CFS for each state ?
P.S. I fought a big insurance company for 6 years to honor my Long Term Care policy for in-home caregiving since I’m 80 and have been housebound with no car for years. I refused to hire an attorney and give them 30% for 5 yrs in case I had to go to a facility at some point. I fought tooth and nail because the ridiculous reason for rejection was that they had NO evidence that ME/CFS/FM is a “chronic illness”. I did 3 appeals each year and finally in January 2025 they believed me and my pile of physician letters, guides from Bateman Horne and a phone call to Open Medicine Foundation. I paid premiums for 27 yrs and figured it was time for them to fulfill their part of the bargain. They made my condition noticeably worse with each rejection.
Finally, regarding inflammation : besides the microglia acting up and not recovering from viruses, I am inflamed everywhere from head to toe. I’m reddish purple in my hands, feet, lower legs and arms, and feel like I’m on fire half the time.
I took 2.6mg methotrexate once weekly for years which got it to settle down, but am now on ZERO medication. I was tired of wearing masks and avoiding people due to it lowering my immunity. Having been an athlete and travel agent, I resented the implication that I was making it all up. I insisted that nobody would CHOOSE this condition.
Thanks for all you do at Health Rising !
No wonder I’m so worn out ! It’s exhausting just to read about all the inflammation. I can actually feel it when it ramps up. I grab a specific place on my head each time Thanks for trying so hard to help us !