

Health Rising’s neuroinflammation emphasis continues. The next couple of years may indicate that neuroinflammation and microglial activation play key roles in fibromyalgia and chronic fatigue syndrome. If that turns out to be true, the attention will probably quickly shift to something called the kynurenine pathway.
Kynurenines in CNS disease: regulation by inflammatory cytokines. Brian M. Campbell, Erik Charych, Anna W. Lee and Thomas Möller. Frontiers in Neuroscience. February 2014| Volume 8| Article12 |

If neuroinflammation turns out to be a major player in ME/CFS and FM the kynurenine pathway will be amongst the first places to look
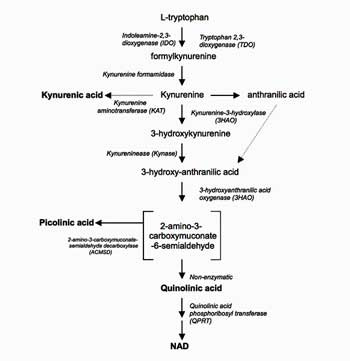
The story starts with the metabolism of a single substance – tryptophan (TRP). Tryptophan is an essential amino acid that forms the basis for some central nervous system heavyweights. Serotonin and melatonin are both derived from it and other tryptophan metabolites have significant functions.
Tryptophan is usually metabolized into serotonin and other factors but in the presence of inflammation, whether in the body or the brain, things change. It turns out that immune activation, in particular interferon-y, a key player in the antiviral response causes an enzyme called indole-2,3-dioxygenase (IDO) to transform tryptophan into kynurenine.
That has the unhappy result of depleting the brain of serotonin but that’s just the beginning of the problem.
The Road Not Taken
Kynurenine metabolism is complex but for our purposes the key fact is that at one point kynurenine metabolism splits into the two pathways – a neurotoxic (bad) pathway powered by an enzyme called KMO, and a neuroprotective (good) pathway powered by the KATS enzyme. Inflammation triggered tryptophan metabolism always sends kynurenine metabolism whizzing down the neurotoxic pathway. A recent review of the kynurenine’s pathway effects in HIV/AIDS called it “An Immune Checkpoint at the Crossroads of Metabolism and Inflammation“. (HIV proteins activate the kynurenine pathway)
Activation of this pathway prods the microglia to produce substances like QUIN, an excitatory agent that sends the NMDA-type glutamate receptors into a glutamate producing frenzy. QUIN also teams up with a substance called 3-HK to produce oxidative stress.
Insights into how the kynurenine pathway may figure in chronic fatigue syndrome and fibromyalgia may be seen in interferon-alpha (IFN-a) trials in hepatitis and cancer. Hepatitis C patients getting this intense immune booster experience similar symptoms (fatigue, depression, pain) and central nervous findings as ME/CFS patients.
Studies suggest increased kynurenine levels in hepatitis and breast cancer patients are associated with increased anxiety.
Another link with possible implications for ME/CFS and FM concerns Epstein-barr virus (EBV). Chronic active EBV infection is present when antibody test results indicate an active infection is present. Most people with ME/CFS do not have that kind of EBV infection, but it’s intriguing that chronic EBV activation – which occurs in many cases of mononucleosis – triggers IDO to degrade tryptophan to kynurenine. Given kynurenine’s ability to sensitize the microglia kynurenine pathway upregulation, could be the straw that breaks the camel’s back for many.
Chronic Fatigue Syndrome and Fibromyalgia
Several studies suggest tryptophan levels may be lowered in fibromyalgia and/or ME/CFS. One study suggested an aberrant tryptophan-kynurenine pathway was operating in FM patients. Blankfield asserts the reduced tryptophan levels could reflect malabsorption in the gastrointestinal tract or chronic infection (or presumably chronic inflammation). The fact that tryptophan degrading bacteria may help produce leaky gut syndrome as well suggests an inflammatory loop beginning at the gut and ending at kynurenine production in the central nervous system could occur. On top of all these kynurenines have profound T-cell-depleting properties.
Treatment Implications
The KP is an important target for the development of new therapies against neurodegenerative disorders, as it is comprised of compounds influencing processes related to excitotoxicity, oxidative damage and inflammation. Bohar et.al 2015
If inflammation either inside or outside the central nervous is triggering the neurotoxic arm of the kynurenine pathway, what can be done about it? Not a lot right now, but the discovery that the kynurenine pathway upregulation is present in virtually every central nervous system disorder is spurring research.
Numerous targets on kynurenine activating enzymes have been identified that could be used to knock the neurotoxic portion of the pathway down. Several compounds have been developed, one of which may be able to stop the production of the neurotoxic metabolite QUIN in its tracks. Other compounds have been developed to turn the neuroprotective side of the kynurenine pathway on.
Compounds being developed to turn off the kynurenine pathway could be an option for FM and ME/CFS patients
Miller reports that recent advances provide “great promise” for the development of IDO inhibitors that stop tryptophan from being degraded to kynurenine in the first place. An IDO inhibitor called 1-methyltryptophan (1-MT) is being tested in mice and in human trials of advanced cancer. IDO is a tricky substance: while it’s upregulating tryptophan metabolism it’s also helping to tamp down autoimmune responses and it’s knocking down natural killer cells. IDO inhibitors may not be useful in those with autoimmune disorders.
They may be useful in depression, however. A recent review suggests further research into the intersection between tryptophan degradation, inflammation and depression will likely “provide many targets for novel antidepressant therapies.”
The key factor with the kynurenine pathway is that it’s a growth field. Any disorder from HIV/AIDS, to cancer, to depression, to cardiovascular disease, that is associated with inflammation, has a stake in learning more about the kynurenine pathway. If the trends hold, the number of PubMed studies published on the pathway will almost double from last year. We should learn much more about this intriguing central nervous system pathway in the coming years.
Check out an update:






I have recently been doing lots of research on this pathway and its pssible connection to many things i deal with. I have lots of papers saved to further explore. Some things I’ve learned is if tryptophan doesnt go the seratonin pathway ( due to stress and inflammatory states) it will turn into quinolinic acid (Quin). This is neuro inflammatory and an agonist to NMDA. It upregulates glutamate an excitory neurotransmitter. Which in high levels can cause neurotoxicity. I can see how this could possibly apply to several things that may be connected to different subsets. A few being POTS, MCAS and EDS 3. (This, of course, is just one of my hypothesis.) Lyme pathogens it appears can also cause more quinolinic acid. One of the best MCAS meds I’ve used is Gastrocrom and it happens to be a mild calcium channel blocker. Qunolinic acid affects calcium channels. Ive read so much about this in the last few days but dont have all the science figured out – yet.
There are things that can be used to help with this conversion into the correct pathway. A few mentioned are – turmeric or curcumin, green tea, and yucca, luteolin (which is from chamomile) If we could get this pathway going in the right direction of seratonin, would that help everything? Should improve mood, sleep, pain, allergies and brain function. If an inflammatory state is one thing that causes the tryptophan to go the route of Quin then addressing this should make a huge diffrence.
One new thing I’m trialing for inflammation and autoimmune and also works on H1 and H2 function and would address MCAS is Black Cumin Seed. Im also using turmeric and yucca. There seems to be a connection to BH4 and Yucca helps to moderate this pathway. It helps the body break down proteins so it doesn’t turn into ammonia. I believe in a vegan diet. For me its essential because of CKD (which I’ve pretty much reversed with this diet ).
If anyone wants links once i get it all in one place I will provide those. Its exciting when we find possible new directions.
Issie
I want those links! (and thanks for treatment suggestions…). This pathway is going to show up – it already has actually. It’s exciting to find a pathway that’s present in many central nervous system disorders…That suggests to me that it may be up regulated in some parts of the brain in some disorders and in other parts of brain in other disorders..or it may be tweaking the microglia in different ways in different disorders. However it turns out treatments are going to be targeting this pathway….I like it!
There are a lot of different circles talking about this right now. Ive been listening to a seminar for the last week and it is being talked about as a contributor to depression. Lots of docs are making this connection. Some are thinking its not a lack of seratonin alone, But maybe this pathway is one contributing factor.
I’ll email you what I have (its a lot) and you can decide what you would like to post.
Issie
Hi Issie. I want to inquire about yucca. I am interested in trying it but I also found it can be poisonous? And it is often confused with something else called yuca? And has several other names. I am a bit confused. Which one is the benefitial one and can it be eaten raw? The reason I ask is because I am recently getting into to raw vegestable juicing.
These may help with your seeing some of the reasons yucca may be of benefit. This isn’t the only thing that may help. If just happens to be the one I’m being asked about and also happens to be one of the things I choose to try myself.
Issie
Yucca benefits
http://www.vitaminsestore.com/yucca-benefits-reviews-side-effects-and-dosage/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1440857/
CBS mutation with Lyme -yucca helps with ammonia
http://www.tiredoflyme.com/ammonia-a-lyme-disease-exotoxin.html
BH4, CBS and ammonia
http://jheldt.hubpages.com/hub/How-to-lower-ammonia-for-those-dealing-with-A1298-NOS-SUOX-and-CBS-mutations
One persons research on ammonia
http://www.gestaltreality.com/2013/07/23/anxiety-ammonia-the-nmda-receptor/
Great on methylation
http://www.herbaltransitions.com/methylation.html
Anti inflammatory effects of yucca and species used
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1440857/
I would love to hear more on this subject as well. Have been dealing with FMS for way too long and this makes a lot more sense to me. Please email me what you have found. Thanks!
i would love links to some of the articles you found, once you get them in order. This sounds fascinating. I’m a marriage and family therapist and work with many chronic fatigued and depressive clients. Thank you 🙂
There is a slight correction to the above comment. CBS mutations cause more ammonia in the body due to inability to process sulfur properly. Yucca helps with this ammonia problem. There is a close tie in with BH4 and CBS in the methylation process.
http://www.gestaltreality.com/2013/07/23/anxiety-ammonia-the-nmda-receptor/
I read several articles connecting high ammonia to CFS.
Here is the other herb/spice I’m trialing – it’s supposed to work like an H1 and H2 to help MCAS. Its also an immunomodulator, and many other things.
http://www.kitchendoctor.com/herbs/black_cumin.php
http://draxe.com/black-seed-oil-benefits/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3642442/pdf/apjtb-03-05-337.pdf
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3642442/
Issie, this is fascinating to me and I’d be grateful if you could let me have the papers / links on your findings. I have no doubt much of this applies to my issues and I’m just yesterday started on cucumin, which although likely to early to tell has eased my pain and the feeling of of ‘swollen brain’ considerably. I’m keen to read everything I can get on this subject and I’m now going to look up yucca, thank you!
Lots are listed here in this blog. I’m jazzed about it, as I think it may be part of my issues too.
Issie
I would love to hear more on this subject. I’ve always read they don’t know what causes my fibromyalgia and chronic fatigue. Finally somewhere to start digging up information. Please email me what you’ve found. I’ve heard black cumin is amazing for inflammation and I’ll be trying this soon.
low-key version of mainstream viruses so they can pass the BBB take over (serotonin, dopamine and perhaps some others-producing) braincells → metabolism comes to a halt, whether it’s because the virus has taken over for so long or the body shuts the cell down itself to prevent apoptosis I dunno (yet) → causing us to feel like we’re on antidrugs (instead of energetic, outgoing, dancing through life we’re depleted couchpotatoes)
can’t make it shorter or probably more know-it-ally obnoxious than this 😀
oh and regarding Rituximab; EBV lives inside b-cells.. coïncidence? moi thinks not
Would St. John’s Wort be helpful for this pathway? I remember reading somewhere that it has cytokine-inhibiting, anti-inflammatory properties, because it contains quercetin.
I’ve been afraid to try it. I could do with boosting my serotin levels, but I worry I might be prone to high dopamine. Could that be explained by poor functioning of the kynurenine pathway?
According to the seminar that I listened to over the weekend, many of the “forward thinking – out of the box” docs are thinking that the meds used may not be the best solution for depression. Not only might they not work, but if they do – they don’t forever. What an SSRI does is inhibit reuptake. With time, what is thought to occur, the body down regulates production as the neurotransmitters are perceived to be in abundance. With time, there is less neurotransmitters because the receptors are blocked from receiving them. (I hope I understood what they were saying correctly.) Then if a persons meds stop working, the meds have to be changed. When this happens, the body has to start producing what has been blocked and that takes time and it’s really hard to get off the meds. St. Johns Wort is considered an alternative SSRI. If it’s true that it blocks the receptors – (my guess is) the results would be the same. May be the same for 5HTP. (One would have to see if 5HTP is a reuptake inhibitor. I don’t want to do the research right now.) However, these would probably be a much better choice than meds. That being said – alternative supplements are meds. The body has to break them down and use them the same as any other medicine. It just may have less side effects.
As for whether it would work on this conversion of tryptophan – not thinking it would. It’s the pathway of conversion that we are talking about. Whether or not inflammatory and/or autoimmune issues are in place and swinging the conversion to quinolinic acid or serotonin. It has to do with balance. We probably need both things. They both serve a purpose. Its just addressing the dysfunction at the core level – autoimmune system and inflammation.
Issie
Wondering if this might explain my weird paradoxical reaction to L-tryptophan, which makes me anxious and disturbs sleep. (I also have most of the symptoms mentioned in Cort’s write-up, including decades of gut problems.)
Looking forward to trying some mitigating treatment possibilities.
It appears that the tryptophan will go down the quin pathway if there is autoimmune issues and inflammation. So, if this is the case – it’s possible. The key is balance. It appears that too high levels of tryptophan could be detrimental too. Some of the research I read this morning showed that some cancers use tryptophan to hide from the immune system. Obviously, there must be some benefit from quin and we don’t want to imbalance the ratios. Key is the autoimmune system and inflammation.
Issie
The seminar I listened to was recommending amino acids to tweak neurotransmitters. But, there has to be caution there too – cause they can go down wrong pathways also. Sometimes there can be paradox reactions (things do the opposite of intended result). There are reasons for these responses. (Like giving tryptophan when there is an issue with pathways.)
Issie
Interesting!…
So, what about taking 5HTP?Considering this information, it would make harm or help?
It would up seratonin but may not affect the tryptophan pathways and may not help with it converting into quin rather than seratonin. What I’m understanding from what is known, there usually is an inflammatory or autoimmune connection for this to go down this pathway. We still have to address both of those things for it to balance out. Inflammation and autoimmune system.
Issie
Neurotoxin with Multiple Targets
http://downloads.hindawi.com/journals/omcl/2013/104024.pdf
same article just not a download
http://www.hindawi.com/journals/omcl/2013/104024/
Associated with OCD
http://www.lapislight.com/wp/tag/obsessive-compulsive-disorder/
Associated with Alzheimers Disease
http://www.maneyonline.com/doi/abs/10.1179/135100002125000550?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&
Associated with Lyme Disease
http://www.drheise.com/ResourcePDFs/Neurodegeneration.pdf
Quin and MS
http://www.jneuroinflammation.com/content/11/1/204
Can affect Calcium channels – interesting that GastroCrom used for MCAS is a mild calcium channel blocker
http://www.researchgate.net/profile/Gilles_Guillemin2/publication/40692615_Neuroprotective_effects_of_naturally_occurring_polyphenols_on_quinolinic_acid-induced_excitotoxicity_in_human_neurons/links/0fcfd511c577c5fea1000000.pdf
Turmeric helps inflammation
http://www.mdpi.com/1420-3049/20/5/9183/pdf
http://massspectrumbotanicals.com/turmeric-vitamin-t-vs-world-disease/
Curcurmin helps with Quin modulation
http://www.jnutbio.com/article/S0955-2863(12)00033-2/abstract#/article/S0955-2863(12)00033-2/fulltext?mobileUi=1
Quinolinic acid connected to Lyme
http://www.clinicaleducation.org/resources/reviews/a-novel-approach-to-treating-depression-how-probiotics-can-shift-mood-by-modulating-cytokines/
http://suzycohen.com/articles/lyme-disease-and-bartonella-more-common-than-you-think/
http://www.lymeneteurope.org/info/vitamin-c-a-lyme-patient-s-friend-or-foe
Quinolinc acid and autoimmunity and depression
http://www.ncbi.nlm.nih.gov/pubmed/1531156
http://www.hindawi.com/journals/omcl/2013/104024/
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2012.08485.x/full
http://www.jneuroinflammation.com/content/8/1/94
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2012.08485.x/full
http://www.jneuroinflammation.com/content/8/1/94
Can affect Calcium channels – interesting that GastroCrom used for MCAS is a mild calcium channel blocker
http://www.researchgate.net/profile/Gilles_Guillemin2/publication/40692615_Neuroprotective_effects_of_naturally_occurring_polyphenols_on_quinolinic_acid-induced_excitotoxicity_in_human_neurons/links/0fcfd511c577c5fea1000000.pdf
Turmeric helps inflammation
http://www.mdpi.com/1420-3049/20/5/9183/pdf
http://massspectrumbotanicals.com/turmeric-vitamin-t-vs-world-disease/
Curcurmin helps with Quin modulation
http://www.jnutbio.com/article/S0955-2863(12)00033-2/abstract#/article/S0955-2863(12)00033-2/fulltext?mobileUi=1
Quinolinic acid connected to Lyme
http://www.clinicaleducation.org/resources/reviews/a-novel-approach-to-treating-depression-how-probiotics-can-shift-mood-by-modulating-cytokines/
http://suzycohen.com/articles/lyme-disease-and-bartonella-more-common-than-you-think/
http://www.lymeneteurope.org/info/vitamin-c-a-lyme-patient-s-friend-or-foe
Quinolinc acid and autoimmunity and depression
http://www.ncbi.nlm.nih.gov/pubmed/1531156
http://www.hindawi.com/journals/omcl/2013/104024/
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2012.08485.x/full
http://www.jneuroinflammation.com/content/8/1/94
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2012.08485.x/full
http://www.jneuroinflammation.com/content/8/1/94
I hope this shows up as links. I copied it all into an email and then copied it. I will send this to you via e-mail Cort and if this doesn’t work – maybe you can fix it. I may have copied some articles twice. But, this will give you a lot to read. Some of it is very technical (I love that.) But, you will get the jest of the meanings and what the implications are. You will be able to see how this “might” could apply to us and many of our complicated symptoms. Key here again is addressing inflammation and autoimmunity. (If these have to be out of order for Tryptophan to go down the pathway of Quin.)
Issie
Wow. That’s great Issie – thanks a lot..
I like science and am intrigued by the complexities and technicalities of the human body. We can never learn or completely understand how awe inspiring our Creator has made us. You learn one thing and realize how that can fit in somewhere else, as you learn more. And why one thing that may be of benefit affects us in other ways we didn’t even know of. Like for example – I knew Gastrocrom helps my MCAS. I found out recently it’s a mild calcium channel blocker. Now to learn that calcium channels may be affected with this quinolinic conversion. This is also connected to NMDA and glutamate – which is excitatory. (I’ve been thinking this pathway has a connection.) It seems this is another piece of the puzzle. It’s exciting when more pieces come together.
Another thing to explore is pyrolouria. Here’s some links: (these links are trying to get you to buy something – but the seminar I heard – the docs treating this are using the questionnaire and telling people to try it and see if it makes a difference) (Of course, me being vegan – I don’t agree that one would need animal products to correct this if it’s an issue.)
http://www.custommedicine.com.au/pyroluria/
http://www.antianxietyfoodsolution.com/wp/wp-content/uploads/2014/10/Pyroluria-questionnaire-from-The-Antianxiety-Food-Solution-by-Trudy-Scott.pdf
http://www.naturalhealthprotocol.com/pyroluria.html
Issie
Low serotonin levels can be addressed by taking the serotonin precursor 5HTP. Serotonin levels along with other neurotransmitters can be measured with urine and saliva by laboratories that are used primarily by alternative physicians. Mine were measured by Pharmason Labs. My serotonin was very low but all other markers were normal. I take 100 mg. of 5HTP 3x a day. This along with treating small intestine bacterial overgrowth (SIBO) has completely resolved my fibromyalgia pain. Since serotonin stimulates gut motility low levels of it can put a perons at risk for developing SIBO, one of the leading causes of fibromyalgia.
Thanks Darden. I’m trying 5-HTP right now…wish me luck!
Darden,
What are you taking for the SIBO and how is it diagnosed?
I tried 5 HTP years ago and it made me feel spacey and a little nauseous.
SamE worked amazingly well for me for energy and body stiffness, muscle strenght and pain. However, it caused digestive problems.
Comments anyone?
The following abstract of a recent (April 2015) paper promises an overview of “current strategies for therapeutic targeting of the {Kyrunine} pathway.” Does anyone have access to the complete document?
http://www.ncbi.nlm.nih.gov/pubmed/25773161
Semin Cell Dev Biol. 2015 Apr;40:134-141. doi: 10.1016/j.semcdb.2015.03.002. Epub 2015 Mar 12.
The kynurenine pathway and neurodegenerative disease.
Maddison DC1, Giorgini F2.
Author information
1Department of Genetics, University of Leicester, University Road, Leicester LE1 7RH, UK.
2Department of Genetics, University of Leicester, University Road, Leicester LE1 7RH, UK. Electronic address: fg36@le.ac.uk.
Abstract
Neuroactive metabolites of the kynurenine pathway (KP) of tryptophan degradation have been closely linked to the pathogenesis of several neurodegenerative diseases. Tryptophan is an essential amino acid required for protein synthesis, and in higher eukaryotes is also converted into the key neurotransmitters serotonin and tryptamine. However, in mammals >95% of tryptophan is metabolized through the KP, ultimately leading to the production of nicotinamide adenosine dinucleotide (NAD+). A number of the pathway metabolites are neuroactive; e.g. can modulate activity of several glutamate receptors and generate/scavenge free radicals. Imbalances in absolute and relative levels of KP metabolites have been strongly associated with neurodegenerative disorders including Huntington’s, Alzheimer’s, and Parkinson’s diseases. The KP has also been implicated in the pathogenesis of other brain disorders (e.g. schizophrenia, bipolar disorder), as well as several cancers and autoimmune disorders such as HIV. Pharmacological and genetic manipulation of the KP has been shown to ameliorate neurodegenerative phenotypes in a number of model organisms, suggesting that it could prove to be a viable target for the treatment of such diseases. Here, we provide an overview of the KP, its role in neurodegeneration and the current strategies for therapeutic targeting of the pathway.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Thank you everyone for put all your knowledge and ideas out there. It is a wonderful community of people who are seeking to ‘help themselves and others’.
My daughter with CFS showed disregulated kynurenate pathways and quinolinic problems on a metametrix test some years ago……it would be great if there could be ways found to reregulate this pathway.
After 7 years of feeling ‘unenthusiastic’ about everything it would be wonderful to see her ‘get a life’, have friends and socialise…..now 19. Became unwell on her 13th birthday so unless she get better in the next few months EVERY single day of her teen years will have been spent feeling like that. So sad. So much to catch up on! As a parent I struggle with her!
Hope to hear about breakthroughs very soon.
I sure hope so. For her and the rest of us.
Take a look at that pyrolouria questionaire. These two things look promising to me.
Issie
Since many of these are things I deal with, the possible connection intrigues me. I hope I found another piece of the puzzle.
Issie
Lipid peroxidation associated with vitiligo (maybe Quin connection?)
http://www.ncbi.nlm.nih.gov/pubmed/15245542
http://www.ncbi.nlm.nih.gov/pubmed/19488773
JAK3 and endometrosis
http://www.ncbi.nlm.nih.gov/pubmed/17631002
JAK3 and mast cell, vitiligo, rheumatoid arthritis, alopecia, etc (medicine being used to target JAK3)
https://en.m.wikipedia.org/wiki/Tofacitinib
MCAS and BH4 and methylation
http://geneticgenie.org/blog/2013/01/31/mast-cell-activation-disorder-mcad-chronic-illness-and-its-role-in-methylation/
*******Brain Tumors, mitrocondrial dysfunction, vitiligo via lipid peroxidation by ROS, increase in glutamate concentrations due to Quin (This one is technical but worth dissecting.)
http://m.cancerres.aacrjournals.org/content/72/22/5649.full
Here’s a part I felt was important
Involvement of the KP in neurotoxicity
In addition to its participation in tryptophan and NAD+ homeostasis, the KP generates a number of biologically active intermediate metabolites (27). Of these neuroreactive KP metabolites, QUIN is likely to represent the most important in terms of bioactivity. QUIN has been implicated in the pathogenesis of a number of neurodegenerative and neuroinflammatory disorders, including the HIV-associated dementia (HAD), Alzheimer disease, Huntington disease, amyotrophic lateral sclerosis, and multiple sclerosis. QUIN has been shown to induce neuronal death in indirect intracerebral administration and in neuronal cell cultures. Similarly, chronic exposure to submicromolar concentrations of QUIN on neurons produces an adverse effect. QUIN is also known to promote oligodendrocyte and astrocytic apoptosis at pathophysiologic concentrations (reviewed in ref.28).
The mechanisms of QUIN-induced neuronal toxicity are multifactorial. These have been well reviewed elsewhere (reviewed in ref. 28). Briefly, one mechanism for QUIN-induced toxicity in human neurons is related to NMDA receptor overactivation with subsequent excessive free-radical–mediated damage, namely mitochondrial dysfunction, DNA damage, PARP activation, and subsequent NAD+ depletion. NMDA receptor activation, followed by excess free-radical production, is also a primary mechanism for QUIN-associated toxicity in human astrocytes, which is a relatively recent finding. The production of reactive oxygen species (ROS) has also been shown to mediate lipid peroxidation. QUIN can also inhibit glutamate uptake into the synaptic vesicle, which leads to excessive microenvironment glutamate concentrations and neurotoxicity (reviewed in ref. 28).
In contrast to the neurotoxic activity of QUIN, KYNA, and PIC exhibit neuroprotective effects. As an endogenous neuroprotectant, KYNA at low concentrations acts as an antagonist to QUIN by blocking NMDA receptor function through its action on the glycine modulatory site of the NMDA receptor (29). At higher concentrations, it acts at the glutamate site of the NMDA receptor (29). PIC also contributes to brain homeostasis by similarly blocking the neurotoxic effects of QUIN (30). However, it has been suggested that the constitutive synthesis of PIC is overwhelmed by de novo QUIN production during neuroinflammation (31). Neurotoxicity would then negate these neuroprotective effects. The exact mechanism by which neuroprotection occurs is unclear, as PIC does not seem to interfere with the glutamate/NMDA binding site on the NMDA receptor (32). Thus, a highly regulated balance in the production of QUIN, PIC, and KYNA is required for normal neuronal function.
Hello.
I am new to this community and to ME/CFS (I’ve only had the specific symptoms for a couple of months). I have however been suffering for over 3 years from something else which I am 100% positive has caused this. I, like thousands of others, suffer from protracted SSRI discontinuation syndrome. Or protracted withdrawal. Like ME/CFS it is poorly understood and not recognised by many doctors. But almost all of us in protracted withdrawal from these drugs have autonomic dysfunction (Dysautonomia) of some kind. Symptoms reported by people in protracted SSRI withdrawal share so many things with CFS. Serotonin is definitely a factor. Once the SSRIs are discontinued (and especially if discontinued too quickly) brain balance is messed up and people suffer for many years after with a variety of symptoms not unlike CFS. A user from the withdrawal forum called Monica suffered severey for many years but recovered and started a blog that is well worth checking out.Here is a post about the connection between drug withdrawal and CFS:
http://beyondmeds.com/2013/07/08/psych-drug-withdrawal-cfs-fibromyalgia/
I don’t know much about all the science of this and I must say I am very impressed with all the scientific knowledge I see here, but I can definitely say DO NOT use SSRI to try in regulate your serotonin uptake. These drugs are very dangerous and can cause long term issues.
Anton
Anton, sorry you have the need to find us. But I’m sure you will enjoy Cort’s and others blogs. There is a lot of knowledge here. When there is dysfunction , it motivates people to action in order to find solutions. Personally, I think it may be up to us to sort it. There is only so much time a doc can devote to pouring over all the info that is out there. It may be that one or many of us will make the connections to allow them to focus in for more help for ourselves. I enjoy research and putting the pieces together.
As for SSRI withdrawal “causing” dysautonomia – not sure that would be the way it goes. Reason I say this is, SSRIs and SNRIs are used to help POTS people with their dysautonomia. Many find relief with their use. So maybe your withdrawal of the SSRIs uncovered an underlying problem that was being masked by the meds. No doubt coming off the meds has a long lasting effect. But if this conversion issue with tryptophan not converting into seratonin rather into the neurotoxic Quin that also affects glutamate which is excitatory – therein could be the problem. That would seem to create a more sympathetic response to the autonomic nervous system. Then we have varying forms of dysautonomia. (Hypothesis – one of many on my part. BTW, I have Hyper POTS.). However that being said, whether or not this contributes to issues with blood flow and circulation that is very much a part of dysautonomia – I haven’t explored yet. Ha! One thing leads to another thing (I just found my next thing to research). So maybe getting this pathway to work correctly will help a lot of possible dysfunctions. Some of my above links show how so many things could be a result of this dysfunctional conversion. It probably still boils down to inflammation and autoimmune. But maybe this is something to help tweak those things. I’m encouraged!!!!
Issie
Hi Issie. Could you explain the theory about glutamate? I have been experimenting with a thing called LifeWave patches. I have tried some for a period that induces glutathione production in the hopes of adressing a suspected thyroid problem and now I am wondering if that has trickered severity in symptoms and onset of post-extertional malaise. First of all is glutamate and glutathione the same thing? Second of all, is glutamate production supposed to by increased or decreased according to theory? Read that CFS sufferers have glutamate defeciency. But also read that too much glutamate related to GABA was the problem. I am confused.
Hopefully Issie with get to this. My understanding is that the prevailing hypothesis is that too much glutamate – an excitatory neurotransmitter – is probably the problem
Thank you Cort. Very interesting. I will stop using the glutathione patches (that are by the way scientifically proven to raise glutathione by up to 300%) immiediatly. If this has an effect on my symptoms I will report back. I really hope they are the reason I started to get this bad.
However, I don’t think this is ALL the problem – just a piece of it. It’s all very complex and this would be too simplistic to blame it all on glutamate – now, I would maybe include the dysfunction with this pathway and Quin and (for me) maybe too high copper (low zinc, B6 and EFA’s) with pyroluria. (There is a blog on this already.) But glutamate and quin are tied together for runners up. And they seem to have connections in the pathways. But, I stick to what I’ve been saying for a few years – I think the core issues are two things: Autoimmune system dysfunction and Inflammation. What caused the epigenetic changes that triggered what we may be predisposed to— like chemicals, vaccines, stress, trauma etc. When we are very complex with multiple things – Lyme and other protozoa, HyperPOTS, EDS, MCAS, vitiligo, alopecia, CKD, FMS, Brain tumor, hypogammaglobulinimia……my list continues —–It can get very complex and mind boggling to really try to analyze when, where and how come. But, it’s fun to try.
—–people you know this is all a researched guess on my part. I’m using info that someone else has written and trying to piece together what may be relative. It just seems like the pieces fit. Will be interesting IF/WHEN a doctor or researcher can sort it and see if I may be right.
I tend to get tuned into something and research it and push the docs to research and see if it fits. The docs at Mayo AZ got frustrated with me pushing them on MCAS (at least that’s what my neuro told me about my immunologist). They even called another doc in from MN to start research on it. And now, us POTsies are getting treated for MCAS. It pays to dig your heels in and push when you really think you have something figured out. Just trying to give you guys encouragement to DON’T GIVE UP. But, have your science and proof of what you are trying to find out. Don’t just go in and say —-so in so said I should find out about this. Doctors need to have a reason to request a test and with insurance it has to be justified. So, have your own research in place. One mistake I made was not having enough peer reviewed articles. Blogs don’t seem to have the same impact. But, if a doc is open minded – it won’t hurt. Just be prepared to drop it off and get back to them later. ****Love you docs at Mayo, AZ**** Thanks for looking into things and helping us complicated ones out!!
Hopefully, all these links will help you guys out. I’m just passing on what I intend to dig deeper into myself. (Some of those above are duplicated. Sorry about that. Cort tried to fix it. But, it’s still a little off. Won’t hurt you to read it twice. My phone – sent it about 3 times and it was a bigger mess. Thanks Cort for fixing it.)
Issie
http://gordonresearch.com/inner.cfm?itemCategory=46861&siteId=502&priorId=0
Glutathione uses a form of glutamate and two other amino acids. Glutathione is needed. They do two different things. The link above will help you learn.
Thanks Cort, for helping here.
Issie
Appears it will affect endothelial function and can be associated with other disorders because of it. (I threw in a few more autoimmune papers associated with Quin.)
Issie
Brain ischaemia and endothelial function
http://healthmetrix.com.br/quinolinic-acid/
CKD (chronic kidney disease), endothelial dysfunction and kyreninine pathway
http://www.ncbi.nlm.nih.gov/m/pubmed/20439183/
Psychiatric disorders and autoimmunity – glutamate, Quin and endothelial dysfunction
http://www.jneuroinflammation.com/content/10/1/43
Cytokines, vitiligo, MCAS, alopecia, arthritis and autoimmune
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3352652/
This subject seems so promising, I wonder why I don’t see more research papers on it in ME/CFS.
Talking to another member here today and a little more research. EPO may have estrogen properties. But there are some interesting connections I’m finding with estrogen. It helps modify the Quin conversion. (Could that explain why those of us who have had a hysterectomy or menopause – seem worse after this? Also for guys – could this be a compensation trying to keep tyrotophan going the seratonin path instead of Quin?). More research to do.
I don’t know seems the pyrolouria idea could be connected to the kyreniene pathway. B6 plays a part in keeping Quin down too. Zinc also moderates NMDA. Seems tied together to me. The other biggie to address with this (and I haven’t talked about it much) is BH4 issues and the need to eliminate ammonia. (I’m using Yucca). This is very complex and the more I learn – the less I realize I know.
I found this great article written by Chandler Marrs, PhD on brain inflammation due to vaccines and its effect due to quin neurotoxicity from the kynurenine pathway. One of the things suggested to help with this is Vit B6. Interestingly this is part of the protocol for what is being called pyroluria. (Too high copper to zinc ratio. Treatment is Zinc, B6 and EPO – suggested by Trudy Scott. Articles and videos are posted.)
http://www.hormonesmatter.com/reducing-brain-inflammation-vitamin-b6/
Here is a little bit of the article:
With the appropriate vitamin B6, quinolinic acid is not the final product of tryptophan catabolism, NAD+ or niacin is, and any damage initiated by quinolinic acid as a natural by-product within this pathway is offset by two neuroprotective factors, kynurenine and picolinic acid. Vitamin B6 is critical for the kynurenine aminotransferase and kynurinase enzymes; enzymes that lead to neuroprotective compounds, kynurenine or picolinic acid. Kynurenine blocks the cytotoxic effects of quinolinic acid by blocking the NMDA receptor, making it unavailable to quinolinic acid, while picolinic acid is the primary metal chelator (remover) in the brain (likely critically important in post vaccine reactions). In other words, vitamin B6 controls the balance between inflammation and anti-inflammation within the brain and the body.
Vitamin B6 may be critical to maintaining the appropriate balance between inflammatory and anti-inflammatory immune reactions
(estrogens inhibit vitamin B6), it entirely conceivable that many of us are vitamin B6 deficient and, as a result, functioning with a constant level of inflammation. Vitamin B6 supplementation, along with the other B vitamins might be warranted.
For those individuals suffering from brain inflammation mediated by disease, medication or vaccine adverse reactions, vitamin B6 might just reduce the inflammation cascades and improve quality of life.
__________________________________________________
Trudy Scott suggests a protocol to treat pyroluria. She recommends starting with zinc (30 mg), vitamin B6 (100mg) or P5P (25mg) and evening primrose oil (1300mg), plus a good multi-vitamin (with manganese and no copper) and a multi-mineral and sometimes additional magnesium. She suggests monitoring your B6 level on the basis of dream recall and your zinc level with a zinc status test that is a taste test with liquid zinc.
Could there be a connection between pyroluria and the possible problems with excess quin?
She suggested Evening Primrose oil and someone asked her about Borage and she kept saying she liked Evening Primrose oil better. I know this type oil has estrogenic properties. Some of us got worse after hysterectomy and menopause. And some of you guys have an imbalance with low testosterone/estrogen balance. What could estrogen do?
______________________________________
Here are some articles showing estrogen playing a role in quinolinic acid.
The results show that 17-estradiol protects hippocampal neurons from quinolinic-acid-induced neurodegeneration by competing with quinolinic acid to bind to the N-methyl-D-aspartate (NMDA) receptor. This would result in a decrease in intracellular free-calcium influx and resultant neuronal swelling.
http://www.researchgate.net/publication/226243313_17-Estradiol_Attenuates_Quinolinic_Acid_Insult_in_the_Rat_Hippocampus
17β-Estradiol Attenuates Quinolinic Acid Insult in the Rat Hippocampus. Available from: http://www.researchgate.net/publication/226243313_17-Estradiol_Attenuates_Quinolinic_Acid_Insult_in_the_Rat_Hippocampus [accessed Jul 7, 2015].
http://press.endocrine.org/doi/full/10.1210/en.2002-220698
THE GONADAL SEX steroid hormone estradiol serves many important functions. In addition to its classical reproductive role, numerous studies demonstrate that it plays an important trophic and protective role in the brain. Epidemiological evidence suggests that estrogen replacement therapy for postmenopausal women is associated with an improvement of some measures of cognitive performance, protection against cognitive deterioration, and decreased incidence of Alzheimer’s disease (1, 2, 3, 4, 5). In addition, beneficial effects of estrogen on the mortality and morbidity associated with cerebral stroke have also been demonstrated (6, 7, 8).
At the cellular level, estrogen is known to exert neuroprotective effects in various model systems. In vitro studies have shown that 17β-estradiol reduces neuronal damage caused by serum deprivation (9, 10), β-amyloid treatment (11, 12), and exposure to glutamate (12, 13). Although activation of estrogen receptors has been suggested in mediating neuroprotection, the complex mechanisms by which estrogen protects neurons against injury are not completely understood.
___________________________________________
What about zinc? What role may this have to quinolinic acid?
Prevention of quinolinic neurotoxicity by zinc
http://www.ncbi.nlm.nih.gov/pubmed/2151937
Abstract
The effect of zinc on the development and evolution of quinolinic acid-induced alterations in the rat hippocampus in culture was studied ultrastructurally. Zinc, although it possesses intrinsic cytotoxic properties, after application in concentrations comparable with those encountered in vivo, was able to prevent typically observed responses after quinolinic acid exposure, either early or late damage to hippocampal neurons. The results further support the concept of a potential protective effect of zinc against the neurotoxicity of particular excitotoxins.
OKAY – so MAYBE there is a connection between pyroloria and kynurenine pathway conversions between tryptophan and quinolinic acid.
Issie
very interesting. Thanks!
Hi Issie
Curious if you’re still researching all this and if you’ve found any connection to “long covid” as the symptoms are quite similar.
Would appreciate your feedback, thanks.
Cort,
I have never seen any studies in direct relationship to this but it is as simple as this. Until 1991, I had only been sick 4 times in my life, measles, chicken pox, strept throat, and human parvo virus b19, a year before I was diagnosed with an aggressive HPV Cervical Cancer. The HPV was a total shocker after 11 years of monogamous marriage. But nine months after the cancer Fibromyalgia started, we just didn’t know the name. 1999 I get a check up, surprise pre cancer of the Uterus, among everything else but the kitchen sink that is found when surgery is preformed. 3 years later my doctors disabled me. Of course I have the Fibromyalgia symptoms laundry list, but my flash point was Cancer, twice. Ever heard that before or was it the virus that caused the Cancer?
I would like to mention here that with a son with schizophrenia and another with depression (and both with high urinary pyrroles) we have found that even 300 mg of P5P a day orally isn’t enough. I believe that this is because the liver uses most of it so very little gets to the rest of the body. We had to go to sublingual – we just opened the capsules and took the powder sublingually. The reaction to the first dose was extreme – a 25 mg dose raised neurotransmitters far more than a 100 mg dose (of P5P) orally as seen by sleepyness (from excess production of melatonin) in our son with depression and our son with schiz was affected as if he had taken an energy drink. This effect wears off when the person’s stores of P5P are adequate,which happens after a few doses in our experience.
As an additional note – the son with schiz doesn’t have low zinc but the other son has low zinc even taking 60 mg per day. So it is a mistake to think that if your zinc levels are normal you can’t have the low B6.
Hang on… the “metabolic trap” hypothesis and the Cortene trials assume the opposite – that there is a high tryptophan/kynurenine ratio + serotonin overload in ME/CFS patients.
Has anyone found any new information on this in the last couple of years? Keen to learn more, but my brain is not up to proper research atm…
A recent study from Australia suggests that Ampligen (rintatolimod) and Rituximab as potential treatments for ME/CFS. The scientific study explores the whole Kyneurine pathway and its connection to CFS and other associated conditions like POTS, the gut connection, neural inflammation and sleep disruption. What is described is an interconnected game of dominoes with systemic inflammation and mitochrondrial dysfunction as I understand it with my limited scientific background. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9276562/