The kynurenine_pathway_in_the_CNS (By-Grant-RS-Coggan-SE-Smythe-GA.-IJTR; Wikimedia Commons)
These metabolic pathways can really be something. Pathways by their nature can be kind of all-encompassing. We could think of the bigger ones like major rivers that send out tributaries that affect multiple systems. Tryptophan and the kynurenine pathways are one of those. In fact, tryptophan is such a potentially important substance for ME/CFS that hypotheses have popped up proposing that either of two diametrically opposed tryptophan issues could be causing or contributing to the disease.
Tryptophan and kynurenine have been under consideration in chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) for over a decade. Now that it’s popped up in long COVID, it’s a good time to take a deep dive into a rather complex subject.
Thankfully, two Australian efforts, “Could the kynurenine pathway be the key missing piece of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) complex puzzle?” and “The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome“, provided a nice start.
Metabolism
Tryptophan is an essential amino acid that we must derive from our diet. The precursor to serotonin, melatonin, and vitamin B3, tryptophan can affect the body in many different ways.
Tryptophan metabolism, or breakdown, can go in two directions – down the kynurenine pathway, or down a pathway that produces serotonin and melatonin. The vast majority of it goes down the kynurenine pathway – the ultimate outcome of which is the production of a crucial energy source, NAD+.
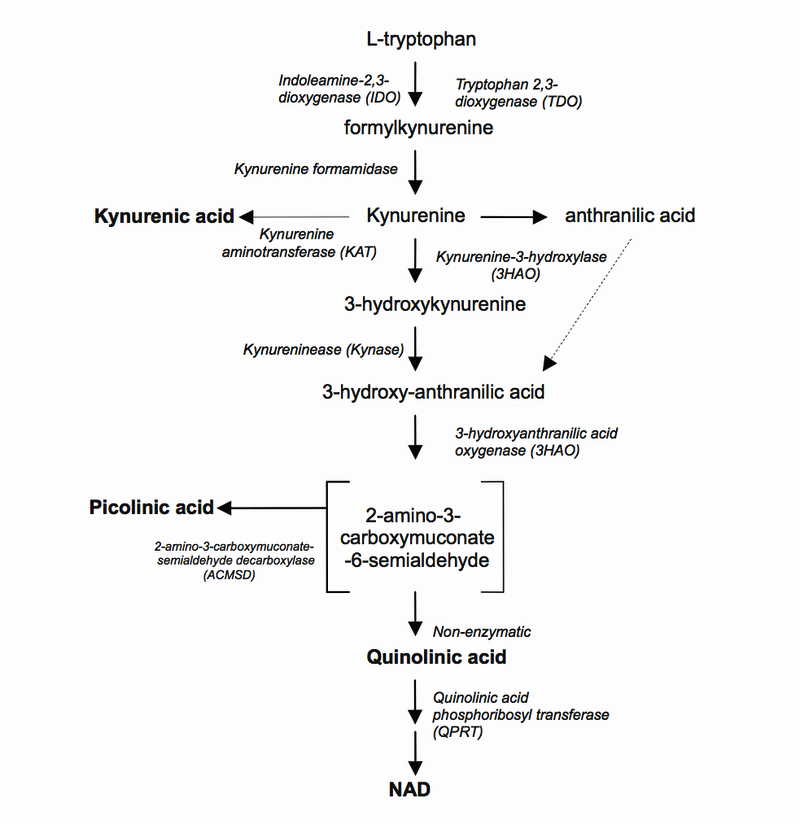
On the way to producing NAD+, though, some bad things can happen. If you want to know the details, check out the paragraph in parentheses below. The crucial thing to note is that of the six metabolites produced in the two kynurenine pathways, four of them (kynurenic acid, (3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3HAA), and quinolinic acid (QUIN)) are toxic.
(Two kynurenine pathways are present:
- Toxic – TRYP is catabolized to KYN which is then converted to 3-hydroxykynurenine (3-HK). 3-HK is then metabolized to 3-hydroxyanthranilic acid (3HAA) and quinolinic acid (QUIN).
- Protective – Kynurenine is converted into kynurenic acid (KYNA), a neuroprotective metabolite that shuts down the toxic metabolite quinolinic acid.)
The Beginning – the Gut
It gets a little tiresome to say, but the problems, as so many do, may begin in the gut. Studies in ME/CFS have consistently shown a less diverse, more pro-inflammatory gut that contains less of the protective butyrate species. Plus, leaky gut – particularly after exercise – has been found.

Gut problems in ME/CFS/FM and long COVID could affect kynurenine metabolism in the brain.
Through its production of helpful substances, the gut has been called the brain’s peacekeeper, but if it can’t keep the peace, problems can happen and some of them involve tryptophan. The digestion of dietary proteins in the small intestine leads to the release of tryptophan, which can be absorbed through the intestines, enter the bloodstream and make its way up into the brain. Tryptophan is also broken down into several compounds (serotonin, kynurenine, indolyl compounds, and tryptamine) that play important roles in gut-brain axis communication.
In fact, most of the kynurenine found in the brain comes from outside of it. Kynurenine is then metabolized in the brain, where it either produces important substances like NAD+ and serotonin, or toxic substances like quinolinic acid. The brain, then, needs a steady dose of tryptophan and kynurenine from the gut to operate properly.
The reduced levels of butyrate-producing bacteria and short-chain fatty acids (SCFAs) found in ME/CFS patients’ guts could result in greater tryptophan breakdown in the gut, resulting in reduced levels of tryptophan. The same is true with regard to the increased levels of Bacteroides bacteria.
Problems in the gut, then, could ultimately result in less serotonin and melatonin being available in the brain.
A Toxic Brain?
It’s important to note that while tryptophan and kynurenine can pass through the blood-brain barrier to the brain, the toxic metabolites (3-HK, 3HAA, Quin) can’t. These substances are only produced in the brain itself – largely by microglial cells but also by astrocytes, and possibly by macrophages that have made their way into the brain.
Two metabolites at the very end of the chain are helpful: NAD+ is an essential energy source and KYNA is a neuroprotective NMDA receptor antagonist; that is – it turns down the activity of the excitatory NMDA receptor which, if overactivated, can turn on the pain producing pathways.
Of all of the detrimental metabolites, quinolinic acid stands out. It’s a nasty compound that triggers the production of reactive oxygen and nitrogen species (RNS) that rip open the lipid linings of cells and damage proteins and even the nucleic acids inside cells. It’s also a neurotoxic NMDA receptor agonist; i.e. it turns on the NMDA receptors found on the neurons, causing a massive influx of calcium ions that can overly excite and harm them. It can also harm the integrity of the blood-brain barrier.
This dual nature of the kynurenine metabolism, with its protective or toxic pathways, led one multiple sclerosis researcher to call the kynurenine pathway, “perhaps the most important regulator of the production of both neuroprotective and neurotoxic compounds” (in the brain).
The Key Factor – Inflammation
But why might this critical pathway flip from producing helpful substances like serotonin and melatonin to toxic metabolites in diseases like ME/CFS, fibromyalgia, and long COVID? What makes people with these diseases potentially vulnerable to kynurenine pathway problems?
In a word – inflammation. As noted earlier, the two major sites of TRYP breakdown in the brain are microglial cells (neuroinflammation) and astrocytes. In fact, the microglia are the main source of quinolinic acid in the brain. Macrophage infiltration into the brain – which Avindra Nath has found in long COVID, and Jarred Younger is assessing in ME/CFS – is another potential source of quinolinic acid. Note also that the gut in ME/CFS appears to be pro-inflammatory, and inflammation in the gut can translate into inflammation in the brain as well.
The toxic branch of the kynurenine pathway is being investigated in numerous neurological diseases
An ongoing neuroinflammatory process in the brain, such as Younger, Herbert Reinz-Polter and others have proposed, could activate the toxic kynurenine pathway and result in the production of high levels of quinolinic acid. One review article stated, “kynurenine pathway activity is correlated with the elevated neuroinflammation in many diseases of the central nervous system.”
Besides producing large amounts of oxidative stress, quinolinic acid could then ramp up NMDA receptor activity, potentially causing an excitotoxic state that causes neurons to flame out – all the while reducing levels of the feel-good neurotransmitter serotonin. That’s a recipe for an overheated, twitchy, pain and flu-like symptom-producing brain.
Maes proposed tryptophan metabolism issues are present in both ME/CFS and schizophrenia. Given how effective low doses of the antipsychotic Abilify have been for some people with ME/CFS, tryptophan pathway issues in the brain could explain why this drug can be helpful in these two very disparate diseases. A recent hypothesis paper that highlighted the “remarkable phenomenological and neuroimmune overlaps” between ME/CFS and multiple sclerosis proposed tryptophan metabolism issues are in play in that disease as well.
The Gist
- Tryptophan metabolism and the kynurenine pathway are so potent that two diametrically opposed hypotheses involving them propose they could be causing ME/CFS/FM and long COVID.
- Tryptophan is an essential amino acid that is produced in the gut by the breakdown of proteins. It’s metabolized in two ways: most of it is broken down in the kynurenine pathways and a small amount is broken down to produce serotonin and melatonin. The kynurenine pathway also produces a vital energy source – NAD+ that is involved in many cellular interactions.
- If the gut microbiome is disturbed – and it is in ME/CFS/FM and long COVID – tryptophan may not be broken down properly. Since most of the tryptophan and kynurenine in the brain comes from the gut, and these factors play an essential role in the brain, proper gut functioning is essential for brain health. Plus, the pro-inflammatory composition of the gut in ME/CFS/FM and long COVID could translate to neuroinflammation in the brain.
- The kynurenine pathway shoots off into two branches – one that produces toxic metabolites like quinolinic acid and one that produces neuroprotective factors like kynurenic acid. These metabolites, it should be noted cannot pass through the blood-brain barrier and are only produced in the brain itself
- This brings up the question of what might cause toxic kynurenic metabolites to be produced in the brains of people with ME/CFS/FM and long COVID. The answer is inflammation. Inflammation in the brain triggers kynurenic metabolism to produce toxic elements like quinolinic acid that produce oxidative stress, harm neurons, impair mitochondrial functioning, and activate the microglial cells all the while reducing serotonin and melatonin levels.
- That’s essentially a recipe for an overheated, twitchy, pain and flu-like symptom-producing brain – making it no wonder that the kynurenine pathway is being investigated in many neurological disorders.
- Reduced NAD+ levels can result in reduced energy production. Another NAD+ pathway called the salvage pathway might be able to help restore at least some NAD+ levels. (See the blog for a link to possible treatments).
- While the evidence is limited in ME/CFS/FM and long COVID in general it points to kynurenine dysregulation in these diseases. Still, much more work needs to be done before we can say that’s happening.
- Two other hypotheses (Metabolic Trap, Cortene) posit that the opposite situation has occurred and that high serotonin levels are causing ME/CFS.
The NAD+ Mitochondrial Angle
The “good side” of the kynurenine pathway breaks down tryptophan to produce NAD + – an essential energy source for the cell. In fact, the kynurenine pathway is the sole source of de novo NAD+ production. While other pathways to produce NAD+ exist, the kynurenine pathway is the most efficient.
With many reactions in the Krebs, or TCA, cycle and glycolysis requiring NAD+, it’s an incredibly important factor. Hyperactivation of the kynurenine pathway could limit the availability of NAD+ and potentially exacerbate the symptoms of ME/CFS.
Decreased levels of NAD+ could affect everything from energy production to calcium homeostasis (blog on this coming up), apoptosis (cell suicide), ageing, DNA repair, immunogenicity and gene expression regulation. In short, whacking NAD+ is potentially a very big deal and some believe low NAD+ levels play a big role in ME/CFS.
ME/CFS – the Evidence
More work obviously needs to be done, but several studies suggest something has gone wrong with the kynurenine pathways in ME/CFS.
Significantly higher concentrations of free TRP in ME/CFS patients, found in 2003 and 2005, suggested it was not being metabolized correctly. A 2021 study found that kynurenic acid – the great protector – was less neuroprotective than expected in ME/CFS/FM. The lower KA/QA ratio suggested that increased levels of the toxic quinolinic acid were present. Finally, a recent study showed that patients with ME/CFS have a higher level of 3HK – a toxic metabolite – and lower levels of kynurenine.
In 2014, Blankfield asserted that both ME/CFS and fibromyalgia “appear to meet the criteria of a tryptophan-kynurenine pathway disorder with potential neuroimmunological sequelae.”
Lastly, Fisher’s finding of reduced mitochondrial functioning in ME/CFS could be a function of an overactive toxic kynurenine pathway that’s reducing NAD+ production by whacking the mitochondria with oxidative stressors.
Increased pain sensitivity is a possibility as well. A fibromyalgia study showed that high levels of tryptophan were associated with reduced pain intensity, while high relative levels of kynurenine (KYN/TRP) were associated with increased pain levels.
Long COVID
The role that tryptophan metabolism may play in post-infectious illnesses, however, really got going with long COVID.
In a study that also emphasized lipid and carnitine dysregulation, tryptophan levels returned to normal in recovered COVID-19 patients but remained low in long-COVID patients. Another study found that kynurenine levels were still increased in long-COVID patients but not in recovered patients up to 4 months after the acute infection. The authors suggested that Epstein-Barr virus reactivation, EBV reactivation, and high IL-6 and kynurenine levels may form a kind of diagnostic triad for long COVID. They proposed that increased kynurenine levels reflect a systemic subclinical chronic inflammation that isn’t picked up by standard immune assays.
Another study, however, found kynurenine pathway metabolite levels returning to normal after six months (but fatty acid problems remained). Still another found alterations in these metabolites in some patients but not others.
A gut study found decreased activity in the tryptophan pathways in long-COVID patients with more gut symptoms. The gut-brain axis next showed up in spades when the same pathways were also found to be decreased in those long-COVID patients with more depression, anxiety, etc. Indeed, the authors pointed out that the proportion of patients with significant mental health symptoms before COVID-19 jumped dramatically six months afterward (from 8-54%) in people with long COVID, and suggested that aberrant tryptophan metabolism could contribute. Note that similar reductions in butyrate-producing bacteria and short-chain fatty acids that have been found in ME/CFS have been found in long COVID (and multiple sclerosis).
Besides problems with fatty acid metabolism, a long-COVID exercise study found evidence of issues with taurine and tryptophan.
The authors of a small but successful long-COVID drug trial of Guanificine also reported that they believe that increased kynurenine levels in long COVID were hampering the functioning of the prefrontal cortex – thus causing cognitive, autonomic, and other issues.
A Different Take
Sending the pathway in the opposite direction could have similar effects. In 2019, Robert Phair produced the Metabolic Trap model for ME/CFS, which proposed a dysfunctional IDO2 enzyme results in tryptophan being metabolized mostly to serotonin instead of kynurenine. Besides whacking energy levels via a dramatically reduced production of NAD+, the Trap would produce an explosion of serotonin in the brain. Interestingly, Cortene proposed that a similar serotonin overload occurs in ME/CFS as well.
Boosting NAD+ Levels
One consequence of a hyperactivated toxic kynurenine pathway is reduced Na+ production. Note that if the kynurenine pathway has gone off the rails and is inhibiting NAD+ production, NAD+ might be able to be boosted using a “salvage pathway” that involves its precursors nicotinic acid (NA), nicotinamide (NAM), nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR).
Find out more about that in the niacin blog.
Conclusion
Evidence for dysregulated tryptophan metabolism in ME/CFS/FM and long COVID exists in several places. Problems with the gut flora could lead to reduced production of core metabolites, such as tryptophan and kynurenine, that the brain needs. Plus, the pro-inflammatory gut found in these diseases could help produce the inflammatory condition in the brain that turns kynurenine metabolism in a toxic direction.
The evidence of neuroinflammation in ME/CFS/FM and long COVID is rather sparse at the moment but is growing, with few studies pointing otherwise. If a neuroinflammatory condition exists, it could turn kynurenine metabolism in a toxic direction, causing it to produce substances like quinolinic acid, which produce oxidative stress, more inflammation, increased pain sensitivity and the symptoms associated with sickness behavior (fatigue, flu-like symptoms, depression). At the same time, it was doing that, the toxic pathway could reduce the production of feel-good substances like serotonin and melatonin, and inhibit mitochondrial activity.
This pathway is under investigation in many neurological illnesses, but with few studies done, researchers are still nowhere near showing that it’s producing the problems in ME/CFS/FM and long COVID. The potential to produce mischief, though, is clearly present.
An uptick in interest in this pathway in long COVID could, if it continues, resolve if tryptophan and kynurenine metabolism is playing a key role in these diseases.

These kinds of blogs take work – but they can reveal new possibilities as well.
BIG (Little) Drive Update
Thanks to the over 500 people who have contributed to our drive we are somewhere around 2/3rds the way to our goal in the last week of our drive.
Dense, technical blogs like this one are tough. They’re tough for me and they’re probably tough for you but they’re necessary, I think, and can also be rewarding as they reveal new possibilities and keep us up to date with what’s going on.
Now that this blog is done (hopefully it’s right… (lol)) it sets a baseline we can return to should this field of endeavor prove fruitful in the future. If you think digging into the technical stuff is worthwhile please support us if it’s appropriate for you.






I have heard about the relationship between intestinal flora and this disease for a long time. I have performed fecal bacteria transplantation 7 times in total, and I stopped without any effect. I have also eaten probiotics and prebiotics, which has no effect on me. For people who have no symptoms in their intestines, such treatment may not be effective.
This is a horribly personal question but what method did you use for the FMT? I understand you had no positive effects but did you have any negative ones?
I have done a lot of work in advance, consulted a lot of papers, purchased various tools, cultured the pathogen of the donor and tested the nucleic acid of Clostridium difficile (to prevent him from being an asymptomatic infected person).
I’m sorry, I can’t explain the whole process in detail here, because it’s too complicated. In short, I used enema.
I performed the correct operation without any effect or any long-term adverse reaction. I may feel uncomfortable after the operation, but I don’t think it is a problem because it gradually disappears. After all, it is something is injected into the intestine, it is normal to feel uncomfortable.
If there is any clinical studies contact me, 8 yrs I have been living in this hell. 🙏
Health Rising will soon provide an easy way to find clinical studies in your area.
Hi Andy, you do not need to explain. I am in the autism community in Colorado, and many parents have tried FMT your way with some success. I personally know a child that had terrible autism, and his parents saved up for the FMT pill after several attempts by enema. He improved tremendously but seems to need to do the FMT pill form about every 2-3 years. Thank goodness that it is coming down in price. I am saving up for the FMT pill. I found a doctor up in Michigan, my old stomping ground, offering this service. I wish you well in your journey.
That’s good. I hope your children can become healthier and healthier. I remember that when deciding the frequency of treatment, I referred to the data from China on the treatment of autism with fecal bacteria transplantation, which is really a good way to treat autism.
hi Donna, may i ask about the FMT pill? i know trials of FMT transplantation but they arenot reachable for me. I never heard in belgium about a FMT pill. where can i get it and is it the same as a real stool transplant? thanks!!!
I’ve also tried FMT 6 enemas and twice daily pills for 7 weeks from Microbioma. No difference in my me/cfs so far.
You weren’t supposed to stop the FMT. Keep going and see what happens!
I had for a long time symptoms of too much serotonin, and Dr. Jin, formerly of the Brain Treatment Center, told me not to overdo stimulation of more. That was 10+ years ago, and I feel like gradually that has resolved and now I may be low in serotonin. The CFS, which I’ve had for most or all of my 70+ years is gradually getting better than it was at its worst, but I have no way of knowing if that’s related to falling serotonin levels.
I tested my serotonin level last year, it’s above normal.
As for the intestines, I rarely have a stomach ache. On the other hand, digestion gives me hot flashes and difficult breathing. My general condition is much better when I’ve purged, like before a colonoscopy, or when I barely ate for 10 days, like when I had Covid last November, or when my osteo is working on the autonomic nervous system and viscera…
Hi Claudine , Just wondered how much your osteo treatment is helping your ANS problems , and if you are in the US / Canada or France ? Do you , by any chance have MCAS as well ?
I wish you a “Bonne continuation “ !
Thank you Caroline, I am indeed in France, I understand that you are too.
I may have SAMA. I have already tried the antihistamines, receptor n1 and receptor 2. At the beginning of the treatment, I found that there was a very slight improvement, but afterwards, I did not find any benefit.
This blog post made me making alot of notice taking! And I’ve saved several of the studies for further reading. I’m in the process of investigating “… but I’m just a little tired and should be able to …” aka FATIGUE.
There is also this about “sickness behavior” that I’ve been fighting in my mind. The physical and the psycholigical and the psychosocial meaning of that expression.
It is a part of my internal struggle to juggle these different meanings and I think that when this is straightened we will become less disbelived.
I have for many years, even before I got sick, been talking about a mental cold when talking about anxiety or light depression states. And all these studies that connect sickness behavior, depression, schizophrenia etc with inflammation in body and brain (which by the way also is a part of the body, have to change that way of saying 😉 ) seems to someway confirm that “mental cold”. 💕
A mental cold is a nice way to put since sickness behavior is apparently all put in motion by the brain…
Thanks again Cort. Has anyone suggested how to correct a “hyperactivated toxic kynurenine pathway”? Are the gut interventions the only suggestions so far?
I’m not up on it but a lot of work is being done in this area. Back in 2015 I wrote
“A recent review suggests further research into the intersection between tryptophan degradation, inflammation and depression will likely “provide many targets for novel antidepressant therapies.” and with kynurenine being targeted in so many diseases I would be shocked if there wasn’t quite a bit going on.
One thing may be simply to get the neuroinflammation in the brain down via microglial inhibitors or via healing the gut (if that’s the source of the neuroinflammation). If I understood it correctly, doing that should stop the activation of the toxic side of the kynurenine pathway and allow the healthy pathway to proceed.
Hi Cort, I followed up on a recent article of yours mentioning PEA (and Luteolin). There is substantial information around that it acts on neuoroinflammation. The micronized form of the mixture is called Glialia and can be easily ordered from the Italian co Epitech.it. I just did. I m bringing this up as it may be of interest for others to try.
1) Lower Oxidative Stress since oxidative stress is why you are all sick. (Pollution, stress, EMFs, poor diet, they all raise oxidative stress. Infections just put you over the top of what you can handle.
2) Take more zinc to increase BH4 production and shift the metabolism of Tryptophan back to making serotonin
3) Take more B2 and B6 to help finish turning Kynuernine into NAD, which is the goal of the pathway.
Kynuernine is not he problem, it is a result of the problem , which is uncontrolled oxidative stress.
And yes, even the bad gut makes oxidative stress!
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6304899/
Which is why some of you find relief from fecal transplants, but you are not solving the fundamental nutritional and environmental issue.
You seem very certain of this Spod!
I take zinc, B2 and B6, have an excellent diet, low stress and live in a low pollution area with no nearby masts etc. So why am I still sick?
(I’m sure you are right that oxidative stress is significant in ME/CFS, but the answer is not as simple as you are stating)
Hi Jay, thanks for your response, I am by no means implying. The solution is simple, I’m only implying the problem is simple.
You could have genetics that demand more of a certain nutrient, and I noticed you didn’t mention selenium, which is crucial. How do you say you’re taking zinc but how much you think? And how much copper? Are you taking manganese which is needed by SOD2? That’s one supplement that helps me tremendously when I need it. Is it because of my SOD2 genetics and some of my mitochondrial genetics.
Also, what about omega-3? I can tell you the difficulty of my diet and curing myself of my MECFS. I eat only fish and no plant oils, but olive oil. The role of omega-3 in transporting things like zinc is crucial.
The only answer to anyone’s chronic fatigue is a personalized medicine. There’s no one answer for everyone, but I believe there is only one problem and that is oxidative stress.
Terrific answer, that makes perfect sense…
Este es principalmente el motivo por el que aquí me encuentro… la quinurenina y el acido quinolinico… están más que estudiados y se sabe que funcionan a la perfección. El problema es pasarlo a medicamento, ya sabes, el tema de que la salud es para los ricos solo, eso es lo que quieren las farmacéutica, hasta dentro de años nos toca comernos los mocos. Si se acaba la enfermedad se acaba el negocio.
Oh wow. As a fan of Phair’s trap hypothesis, I’d not realised there was such a history of kynurenine research already. Let alone the new LC stuff. Kinda thought it was out of the blue…
And of course there’s seemingly contradictory findings of associated excess and deficient tryptophan levels, etc. lol!
Thank you, Cort! I am sure that we need to closely follow the kynurenine pathway in ME/CFS and that it may be a key process in the pathobiological matrix.
However, I am not sure if your assumption is correct that “it all starts in the gut”. I know that this has gotten very popular and that there is some evidence going for this. But I want to remind ourselves that so far we do not know if the gut processes (leaky gut, changes in microbiome etc) are really primary processes or if they reflect upstream pathology.
Therefore I would like to again make a pitch to not forget about another known of the elephant in the room, and that is viral reactivation. I do think we should always try to think abnormal findings in ME/CFS (like: an abnormal tryptophan-kynurenine metabolism) together with what we know about viral reactivation. So my question would be: could the abnormalities you describe in this blog be a reflection of ongoing viral reactivation?
And indeed they could – find a review paper here: “Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests”: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4527356/ . It also appears that IDO2 may be a key regulator of viral reactivation (https://pubmed.ncbi.nlm.nih.gov/15374002/ )
Interestingly – and in celebration of the richness of your blog – you had a very interesting discussion in your first blog on the kynurenine-pathway back in 2015 or so, called “Neuroinflammation II: the Kynurenine Pathway in Fibromalgia and ME/CFS” (https://www.healthrising.org/blog/2015/06/28/neuroinflammation-ii-the-kynurenine-pathway-in-fibromalgia-and-mecfs/ ). Here you gave a concise summary of what may happen in HIV-infection:
“Inflammation triggered tryptophan metabolism always sends kynurenine metabolism whizzing down the neurotoxic pathway. A recent review of the kynurenine’s pathway effects in HIV/AIDS called it “An Immune Checkpoint at the Crossroads of Metabolism and Inflammation“.
You also refer to chronic EBV infection, which apparently triggers IDO to degrade tryptophan to kynurenine. I think that it is possible that this also applies to EBV reactivation (or reactivation of other human herpes viruses) and that IDO may even play a role in the switch from latent to lytic viral activity (a switch which may be overactive in ME/CFS).
So interesting! Thanks Herbert. I didn’t mean to suggest that it actually all starts in the gut – just that the gut could be involved either via problems with tryptophan metabolism or as a source of inflammation. That’s really interesting stuff about viral reactivation. Once again, I completely forgot I wrote that older blog – darn! I could have used it (lol).
Video Update from Rob Phair, PhD!
The Itaconate Shunt Hypothesis for ME/CFS
Janet Dafoe interviewing Rob Phair
From OMF celebrating 10years link inHR blog
https://www.healthrising.org/blog/2022/12/20/celebrating-open-medicine-foundation-10-years/
https://www.omf.ngo/video-update-from-rob-phair/
Rob’s next look at the problems with ME/CFS /FM biochemistry gone awry. From what I recall Phair was highlighting all the possible pathways in the Itaconate shunt hypothesis and the problems with CoA production and the competing use of both GABA and glutamate as amino acids as fuel and as neurotransmitters in the brain; the big problem in the brain is that you can only make energy in braincells by using amino acids, because glucose can no longer get into the Krebs cycle, and because you are using amino acids to make energy, you have fewer amino acids to make nerurotransmitters;…… very likely resulting in brain fog……. inability to do cognitive work which depends entirely on glutamate and GABA, Rob & Chris Armstrong also think that glutamate is going to be used by GOT2, GABA prodn GAD & GDH enzymes. GDH is activated by ADP, so activities like thinking or physical, muscle contraction, pumping ions across a neural membrane, any action requiring ATP for energy is going to move the balance from ATP to ADP. So any of these activities that induce PEM will convert ATP to ADP and that is why they think it is important that GDH is activated by ATP…….That means that when you do other activities or increase your level of activity, youre going to convert some of the glutamate to 2 oxyglutarate, and in the process make ammonia, a known neurotoxin
How the
Correction: GDH is activated by ADP
Rob Phair preciseed ‘ADP is produced from ATP by ‘activity’ so when you do activities or increase your level of activity, you are going to convert some of the glutamate to 2 oxoglutarate and in the process make ammonia which is a known neurotoxin, and so we think that its possible that this pathway will explain the PEM phenomenon’
Janet Dafoe ‘Wow’
Rob: ‘So this is what I wanted to tell you today.This is how the Itaconate Shunt, driven by the Innate Immune System; that’s the ancient immune system, can turn an infection, which we often think of as one of the many ways that CFS gets going, can turn an infection into CFS. (Leading to brain fog & PEM)And we have to test this ,obviously.
Whether the TCA cycle is in fact shunting cisaconitate on each ?shunt?
And then finally it raises the question of what makes it Chronic?
Taking you back to an earlier diagram, that the’ innate immune trigger’ is generating the CAD, the enzymenthat starts the Itaconate Shunt , and thatnwould be fine, and its certainly true that it happens, tat’s well established, but it shouldn’t be true in a chronis sense.
It should happen for maybe a few hours, at most a few (4) days, while the innate immune system is limiting the ability of a virus to ‘reproduce’ for example; and it will stop doing this the moment theAdaptive Immune System can take over and deliver antibodies to that virus, that will stop and clear the infection.
So there is something missing in this theory, so far, and that’s the topic of the next session; which is ‘how can this shunt, this production of the enzyme CAD, and the Itaconate Shunt, How can that come back to be a chronic element of human metabolism? Why is it that it does not turn off when the Innate Immune Systemis supposed to have stopped working? And we have ways that we think we can test those ideas, And that’s what I will talk about next time.
Janet Dafoe: Does this enable you to predict the severity levels of different patients?
Rob: We can talk about that next time, but I think that if the hypothesis istrue, you would suspect that the more cells in your body that are running the Itaconate Shunt, the worse you will be; the more severe will be your disease, we think we can measure some of the molecules in the pathwaythat we believe will make it chronic. Some of the molecules should be elevated in p-atients, and they should be more ellevated in severe patients than in less severe patients……….
We all know that the immune system is an important part of the pathology of ME/CFS.
……..And Both the IDO Metabolic Trap and the Itaconate Shunt are directly connected to the immune system in the human body. So the interferon alpha, the cytokines that are doing the signalling is an important part of the innate immune system, and the fact that it turns on IDO as well as CAD……… But it’s probably just because the IDO Trap is directly affecting the immune system by regulating the AHR, (Aerial Hydrocarbon Receptor), which is again sending signals to the nucleus and upregulating genes. So they are closely connected, but I don’t think you need to think about IDO in the same context as the Itaconate Shunt. These are 2 hypotheses, both of which coud be true, ………. The IDO Theory is published, and the Theory on the Itaconate Shunt is my current project. So we need experiments to test, and that’s what is going on in experimental Labs like Ron’s and Chris’s.
So that’s the relationship between the Itaconate Shunt & the IDO Metabolic Trap……….This is the most complicated project we’ve ever worked on…….
Models & Experiments.. that’s what we’re doing… Theory & Practice.
Janet Dafoe: See you in part 2
Fascinating. That Robert Phair – he is really something! Thanks for sharing this 🙂
Can someone elaborate more on the comment re how LDA abilify is thought to be helpful? Was it something to do with tryptophan?
Esa porquería es para la esquizofrenia, es un antipsicótico
Just to put in my 2¢…
I ve tried NAD supplements and just one pill set me off with the worst symptoms. Vomiting, nausea, feeling poisoned, weak, Tachycardia, short of breath and more.. Horrible feeling. WON’T be trying that again! I have not tried Abilify yet, so I might mention that to my Dr. Of course I’m always willing to try ‘again’ to increase the butyrate and short chain fatty acids in my gut…
If you are not on twitter you might not have seen our @remissionbiome MECFS self-experiment. It tests EXACTLY this hypothesis by trying to re-create a temporary remission event both two of us actually had. I am linking to our gofundme for people who are not on twitter but I would really recommend you all check this out. Two of us went from severe to moderate or moderate/mild from a temporary remission event such as the ones we describe.
https://twitter.com/remissionbiome
https://www.gofundme.com/f/Remissionbiome
https://docs.google.com/document/d/1IxmUQPmIlnO0ZnCMxzZfGY9A8mWDwhU-4V2I9-uJULY/edit
I tried Amoxiclav as u said but it didn’t affected my CFS even 1%
Hi Anish,
There is much more too it than just taking AmoxClav. Check out our protocol. You data though is really valuable to us, we need to track negatives as well as positives. I hope you follow us on twitter so that we can keep you updated.
Please see: https://docs.google.com/document/d/1IxmUQPmIlnO0ZnCMxzZfGY9A8mWDwhU-4V2I9-uJULY/edit
Hi Tamara, I notice you are using mictoglial inhibitotrs. I’ve had an opportunity to try these since I found I have lyme recently. I came down with severe me/cfs right after the covid vaccine. (I had me/cfs befor a decade earlier for 3 years that resolved with different therapies that no longer work for me.) Anyways, I noticed an effect of all 3 antibiotics although it was very mixed and I couldnt continue. I feel for sure the microglia are involved in my disease but it makes me feel that the microglia are activated for a reason. I think it could be a susceptibility to mitochondrial dysfunction plus a pathogen. I recently found acetyl co a dehydrogenase deficiency or SCADD in my genetics.
tamara can i have your email please… I want to talk this thing in detail. I also belive my CFS is microbiome related
How about the Enzyme Bh4 ? Tetrahydrobiopterin deficiency? Which also controls tyrosine, phenylalanine and serotonin and thyroxine? Excess phenylalanine is toxic to the Mitochondria
I wonder whether some part of this process is involved in the more recent substantial improvements I’ve experienced. I’m aware of this area but I don’t fully understand it. My view is that personally, I don’t have to understand it because my body is continually involved in processes that it sorts out itself, whether I understand them, or not.
However, having said that, I have been attempting to create the conditions in which whatever systems were all wrong, may right themselves. I get a bit bored of myself because I say the same things but I’m familiar with many of the ideas in this blog, I just can’t understand the more detailed science stuff.
For years now, my focus has been on my gut, diet, supplements and their effect on my brain etc., attempting to calm my sympathetic nervous system dominance and deliberately enhance my parasympathetic nervous system, which impacts my immune system, improve the quality of my sleep, and so on. The usual things. I think it was all important and a good idea.
At some point last year, I started to have some great, restorative naps in an armchair, in the afternoon and in the evening/night. Part of me thought sleeping in the chair wasn’t a good idea; that I should go to bed properly. But then I thought no, these sleeps are great, really deep, it doesn’t matter that I fall asleep and wake up at midnight, 2am, or whatever.
One afternoon I had a nap, where I felt sweaty and as though my illness (whatever that was) had ‘broken’. I started to feel like I was in more familiar territory, like I would if I’d had a nasty flu and it was about three weeks later into my recovery. Over the next few months, I began to feel more normal. Which may not sound very exciting but to me, I could feel something significant had changed.
I’ve been struggling since 2007 and had become much worse in 2016/17 but have been improving slowly since early 2019, or so. However this recent improvement is a shift somewhere and I’m not sure what’s happened but I know that I feel different, in terms of energy availability. I still have extensive issues with food intolerances and I can wind up my brain but on the whole, I feel so much better 🙂
I wonder if there is connection between the serotonin syndrome and Me/CFS/FM.
A B6 defficiency, excess estrogen, cortisol and steroids can all shift metabolism of tryptophan away from NAD+ towards serotonin and the toxic metabolites. [See the work of David A Bender]
This also means a tendency for your body to turn into reductive state, and then reductive stress.
In Naviaux’s rodent experiment injecting ATP, B6 shifted out of cells. So did B1 and B3, from what I recall.
Hypothyroidism tends to lower alkaline phosphatase [ALP], which can affect B6 metabolism. It would make sense to address thyroid health. See Broda Barnes, Ray Peat, Kenneth Blanchard, etc.
I include a note to be very careful supplenting B6. Many don’t do well with it, and most take waaaaay to much [see B6 toxicity]. See the work of Katherina Dalton – she who coined the term PMS – regarding proper dosing, and how increasing protein and carbs alone helped many women, without even needing to use progesterone (nor B6).
The serotonin myth… See David Healy’s article on the British Medical Journal. Ray Peat’s articles on serotonin provide many scientific references. It’s high in hibernation, by the way, and ME/CFS has been likened to a hibernation state.
If you for a second think tryptophan is good – see eosinophilia-myalgia syndrome. They blamed it on contaminated batches of tryptophan supplements, but there are many case studies and reports of it still happening and to people not even ingesting supplemental forms. Tryptophan was banned as a supplement for a while, and it really should be banned again, and the other derivative supplements.
– – – – – – –
I finally developed the rash known as pellagra last summer. I laughed remembering Ron Phair saying in a lecture that he didn’t think the shift of tryptophan away from B3 formation was a big deal [since you can get B3 through your diet].
I read some of the medical treatises when pellagra was a thing, the Italian and French work and later the US ones. There was a very different understanding then, borne out of the very direct experience of working and treating people with it. Doctors lamented that they could spot a pellagrin years before the acute severe attacks, and if only that was better known, the worsening of the person could be avoided. I then understood how I have been sick with pellagra for so long, and in fact, the year I got sick with EBV which led to the severe ME/CFS state – I was experiencing pellagra: the rash was a lot milder then, and in fact, they knew quite well you could have it without the rash.
My favorite quote from one of the doctors, in regards to pellagra:
“There is no disease, only the deseased.”
[It’s presentation was so varied…]
That knowledge has been lost to history. Today a doctor in a developed nation will not recognize pellagra if it were standing in front of them.
Pellagra in the States was largely treated by a diet high in proteins and carbs. Reminds me of the note I wrote above about Dalton’s approach to treating her patients…
One of the effects Rapamycin has is to improve the NAD+/NADH ratio.
Hi, M have you been treated for Pellagra yet & is it 300mg? Yes, potatoes & meat are another way treated in the USA. Potatoes have a very high ratio of B-3 in them
The medical guidelines say 300mg total divided through out the day. I needed between 1 to 2 grams the first few days to stabilize me.
The problem is that any little stress kicks it off again. And exposure to the sun. Basically ‘crashing’ and a bout of pellagra are the same thing for me.
The old medical literature covers all this, it’s not like you take B3 and are fine. Exposure to the sun even after recovered can make it happen all over again.
If I eat corn, it triggers a severe pellagra ‘gastritis’.
Dark chocolate triggers the rash. There are studies on how higher leucine in relation to tryptophan and oyher amino acids is a trigger.
B1 also triggers it, and this seems to include anything made with enriched wheat flour [it has B1].
Low B6, high estrogen, high cortisol and exogenous steroids all can be triggers.
One doctor in the States treated it with hydrochloric acid.
How does B1 deplete B3?
I don’t know. I don’t think it is a linear relationship either. Once you run low on one nutrient, it affects others.
You don’t know how your body adapts for the deficiency, and which processes are stressed, etc.
And it might be different for each individual.
Pellagra is still seen and recognized in parts of India. It seems there it is common to treat with B6 as well. This they gathered from clinical experience.
I could say that B1 ramps up metabolism, and when you ramp up metabolism – any deficiency becomes more pronounced.
But
I no longer need to take B3 daily, but if I take a little B1, I don’t feel well. I’ve tried different forms of it.
However if I take a supplement of most of the B vitamins together, I feel fine.
Dr B1 (Lonsdale) and Dr Constantini and Stasha Gominak all have patients take all the Bs. That’s also from clinical experience, not some textbook pathway thing.
I would venture that many with ME/CFS are pellagrins. The older medical literature mentions suspect pellagra in any one suffering from neurastenia for years. That’s code for hypothyroidism, which is also mentioned.
When I was very sick, any nutrient in supplemental form had a huge effect in tiny doses. B3 and progesterone would do nothing for me. I later learned, because I needed much much bigger doses.
It’s easy to try and see what effect it has on you. Or you can eat liver, etc.
Niacinamide
(NOT niacin. Even the doctors get this right)
That’s really interesting that B1 can make it worse. I feel like it amplifies the feeling of being electrocuted in my legs when I megadose. I am deficient in all the B’s particularly B2 and B3 and B6. B6 worsens pins and needles and numbness but I am sill low in it according to the test. I also have very high serotonin markers on the NutrEval. I also have high quinolinic acid, possibly it’s from lyme and viral reactivations. Why is nicotinamide better than niacin? I recently read nicotine can protect neurons from quiniolinic acid through the nicotinic acetylcholine receptors. Perhaps this is why it helps some people in long covid. Are you saying that quinolinic acid can be lowered by B3? I don’t think lowering it will somehow make the lyme flourish. I imagine other mechanisms in the immune system are being suppressed and out of balance.
If something makes you hnwell when you take it it would be wise not to.
I never did the megadoses. I never did ‘normal’ doses. I don’t know how people do… Which – what is normal?
I took very small amounts, B3 was the only one I did larger doses and that once the pellagra attack was very evident.
B6 is *not* like the other B vitamins. Forget the water soluble non-sense. Look up Katherina Dalton’s articles on her clinical experience using B6 with women. 10-20mg max. She actually worked with the UK government in setting that limit for the sale of supplements decades ago.
And she also found that for most women a change in their diet was enough – more calories, more carbs, more proteins.
Many with hypothyroidism [a.k.a. ME/CFS, post lyne, fibro, etc] don’t tolerate even miniscule amounts of B6.
Which test is showing low B6 – blood PLP or a ‘functional’ test like the OATS, where it is an indirect measurement by looking at the activity of an enzyme? If it is this one, then be aware that excess estrogen / low progesterone can also affect this enzyme/measurement. David A Bender studied this in his lab and published; sadly you get diagnosed with low B6 when it’s the estrogen…
If it’s the blood PLP test – was the test tube covered and shielded from light at all points, evrn when blood was being drawn?
If not, the light interacts and degrades the PLP in the sample, so your values come out lower than they are.
My B6 was high in blood tests, but symptoms were of deficiency.
I got better by reading Ray Peat’s articles and books and trying things out and making changes to my diet and way of living (i.e. more sunlight early in the day, etc).
I know of people that with just dietary changes, they got better. For me it was different, and I had lots of hormonal and nutritional deficiencies that food just didn’t cover initially. I did different things to bring serotonin down – your environment can help a lot there.
Would Cell Danger Response explain B6 not getting into the cell? Membranes pretty much impermeable to everything?
Lots of people seem to be healing from MECFS with nervous system regulation…ATP can ship away from danger molecule service to energy producing service…
For those who like to read, I highly recommend the 2015 book entitled Targeting the Broadly Pathogenic Kynurenine Pathway. It covers a wealth of knowledge on kynurenine pathways not only in human cells, but also some bacteria and fungi that can synthesize and/or process kynurenine. There are entire chapters that have convinced me that IDO2 (alongside IDO1) is not enough to represent the full dynamics of human kynurenine pathways: TDO2 might be just as crucial. Different human cell types have different affinities for IDO versus TDO, and the book covers a great primer on the whole picture. I hope to someday write about an analysis of open source Human Protein Atlas data that I have been working on, which should be able to answer questions on cellular specificity for kynurenine (as well as itaconate) pathways.
I’ve been feeling for a while that processed sugar is very beneficial for myself. It definitely helps me get through social settings more easily.
But beyond that, when I regularly have a sweet dessert after evening meals I feel less symptomy the following day, more motivated and energetic. Still constrained by ME/CFS, but with more like a 50% battery.
I typically operate at around 15% so it’s quite noticeable to be closer to 50% and it enables me to participate in life a lot more.
It’s only a vague notion and I haven’t really looked into it a great deal, but I did read the other day that consuming sugary foods after meals can impact tryptophan in some way. Either by increasing the production or utilisation (or both?).
Going to spend some time over the next few months researching to see if I can put together some sort of testable hypothesis.
If you like how sugar makes you feel, you might like reading Ray Peat’s articles [on his website].
This comment is very timely for me, as I had the exact thought earlier this week.
After being off sweets for several months, I had a craving after a meal and ate a couple pieces of candy. I felt noticeably more energetic and mentally clear all evening and the next day.
So, according to labs done in France, and then a full Genova OATs lab, I don’t have alarmingly high kynurenic acid (I have normal amounts). However, I have LOW gut serotonin (the urinary marker, serotonin metabolites). This went along with low gut dopamine.
However, I have 2 excessively HIGH serotonin markers, from two separate labs, measured at two very different points in time, one of them being high Indican (a bacterial metabolite associated with problems processing/digesting tryptophan) as well as high 5-OH-indoleacetic Acid (not crazy high, just in the yellow zone).
But both of these serotonin markers being raised as well as there being LOW gut serotonin means, to me, that my gut is unable to adequately produce serotonin. The addition of low dopamine means gut is unable to adequately produce neurotransmitters in general.
Too much still unknown.
Are the toxic elements our bodies produce a way of attacking such things as EBV (Prusty) or spirichetes/or neural larval migrans (Dr. Alan MacDonald).
The toxic metabolite like quinolinic acid ‘s name sounds similar to quinoline.
From online “Quinoline has been found to possess antimalarial, anti-bacterial, antifungal, anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory, and analgesic activity.”
Thanks for the article! Very interesting summary.
Sometime within the last couple years, Ron Davis warned patients not to experiment with treating the hypothetical Metabolic Trap because it could lead to incurable autoimmunity. Would you consider adding a note about that to the article? I’m concerned someone may try to act on this by taking tryptophan or similar. I was going to add a link to a source, but I just realized it’s an article you wrote for the OMF, lol. A lot of people probably know about that statement by now, but maybe it would be prudent to mention it in case someone comes across this who doesn’t? (Apologies if I’ve missed it somewhere.)
I’m excited to see where this line of research leads. It sounds promising.
“KYNA & QUINO can’t pass the BBB” this article states.
Now wondering why both (!) are too high in my blood?
I also know “albumin serum/csf ratio” is too high. So blood-cfs barrier could be compromised.
If quinolinic acid makes it to your bloodstream would this mean BBB is compromised too?
Estrogen excess and a B6 defficiency can both shift the metabolism of tryptophan away from NAD, towards the foormation of the toxic compounds you mention.
See David A. Bender studies.
[OjO: it doesn’t imply to supplement B6, rather look into how to improve your metabolism via foods and hormones. The low albumin can be hypothyroidism]
Thank you for your reply.
I read your posts about b2 & b6 and will try them.
The thing about albumin is this:
it is ONLY made in the liver.
It’s normal to have it in your blood (sometimes too high, other times normal in my case).
What is not normal?
When albumin is found in higher concentrations in your csf (spinal tap) than in your blood.
The molecule is too ‘big’ to pass the brain-csf-barrier and it is never a product from your brain itself.
That’s why it’s tested in blood and csf at the same time.
Normal level “Blood albumin” “BCSFB not intact”?
You can improve your metabolism and nutrition by the foods you eat.
Have you tracked your heart rate and temperature, upon waking, an hour after lunch, midafternoon? Etc
How to bring estrogen down and increase progesterone, reduce serotonin, amonia, lactate.
Ray Peat’s work has been very helpful to me.
Be careful with B6. Katherina Dalton found 10-20mg, many with our weird diseases can’t handle it.
Thanks, M for the response, do you recall the name of the Doctor in the USA who treated with hydrochloric acid or dose?
You mentioned 3 items of chocolate corn wheat flour I think all these are high in Nickel or other metals.
Do you have a nickel allergy? (SNAS) Systemic Nickel Allergy Syndrome or Cobalt.
Nickel is in Soya added to white fillings & the silver one has nickel as well
The doctor was mentioned in “Clinical Pellagra” by Seale Harris.
And Henry Fauntleroy Harris goes over the different people who found low hydrochloric acid (and low pepsin) in his book “Pellagra” [1919]
Both have a good overview of the history of pellagra and what the different people involved in different countries and throughout centuries thought/discovered about it.
In was treated with an improvement in diet – of more proteins and change of carbs [from maize]. Just supplementing B3 , without changing your diet, sounds a little nutty to me. Together with the problems in diet, sun exposure triggers it. There might be many people with it that because they avoid the sun, are too sick to go out – don’t develop the rash, etc.
The role of leucine, in the absence of other amino acids and a diet low in protein/carbs, has been established in pellagra. Untreated corn and dark chocolate are high in leucine. Milk chocolate doesn’t trigger pellagra for me.
thanks, M appreciated
Some of you may be interested in joining the FMT groups on Facebook, where many are trying FMT for ME/CFS. Some have recovered a great deal.
Daffodil123, what is FMT
Fecal Microbial Transplant. People saying they did not get improvement from 7 – 8 times doing it…this is normal for many. I myself do not notice improvement until months into it. Some are finding they must do it continually to maintain. Some are in remission and need boosters. Some lucky few get engraftment. You cannot tell anything from a few treatments.
Topical niacinamide. Provides a significantly noticeable relief from brain fog. It’s the ONLY genuine relief I’ve encountered in 12 years with severe CFS. Hopefully the kynurenine pathway involvement recieves greater attention soon. Thank you for the write up Cort!
https://www.youtube.com/watch?v=5wBSdEkQ2cE
Thanks, Karyn have you ever tried the B-3 3x100mg & the Pellagra diet Meat Carbs