
Bindu Paul was recruited to work on ME/CFS by Marian Lemle. A Solve ME grant is funding her hydrogen sulfide in ME/CFS work. (Image from Johns Hopkins University).
Bindu Paul Ph.D. is on a roll. A protégé of the pioneering neuroscientist, Solomon Snyder, Paul is an expert in redox signaling who’s written paper after paper on its role in central nervous system diseases. Paul received an invitation to write a review article on redox imbalances and disease for the Proceedings of the National Academy of Sciences (PNAS) journal last year. PNAS, which has been around since 1914, is one of the most respected science journals around. Since PNAS invites only the best to write review articles, getting that invitation was quite an honor. Just this year Paul was also appointed to the tenure track at Johns Hopkins and is starting her own lab.
Happily for us, Paul, who’s been funded by Solve M.E. to study hydrogen sulfide in ME/CFS, chose to focus her PNAS paper on redox reactions in ME/CFS and long COVID. The way Paul came to ME/CFS is worth telling. Marian Lemle’s story will be told elsewhere, but suffice it to say this citizen scientist, and mother of a daughter who had ME/CFS, took her interest in hydrogen sulfide in ME/CFS to such heights that she authored a 2009 hypothesis paper: “Hypothesis: chronic fatigue syndrome is caused by dysregulation of hydrogen sulfide metabolism” (Check out Marian’s website).
That was just the beginning. Tony Komaroff advised Lemle to get in touch with the experts in the field, and so off she went, getting in touch with Bindu Paul and Solomon Snyder. Bindu Paul accepted her invitation to attend the NIH Conference on ME/CFS in 2019 and they’ve been communicating and collaborating ever since. They began work on what became the PNAS paper over a year ago. After Tony Komaroff offered up a number of insightful ideas, he was invited to participate.

Marian Lemle invited Bindu Paul to study hydrogen sulfide in ME/CFS.
Bindu Paul has long collaborated with Solomon Snyder. One of the few academics to have had an entire Department named after him (The Solomon H. Snyder Department of Neuroscience ), Snyder reportedly has the highest h-index (productivity/impact rating) and is one of “the 10 most-often cited biologists“. Snyder has received the Lasker Prize, and is regularly rumored to be in the running for the Nobel Prize.
Together, this diverse group has produced a novel slant to ME/CFS and long COVID that could bear real fruit in the future.
The Paper
There’s no fudging, no question marks, no hedging in the title “Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome“. It doesn’t say a redox imbalance “might” or “could” link the two diseases together; it states that they are linked together in this way. Indeed, Bindu Paul and Marian Lemle told me that they scoured hundreds and hundreds of publications, and even supplementary materials, to arrive at the data in the paper. It’s a solid piece of work that is intended to serve as a roadmap for those interested in this aspect of ME/CFS and long COVID.
The short takeaway is that the same major redox processes and key pathways appear to be dysregulated in both ME/CFS and long COVID.

The loss of an electron leaves this atom highly unstable. It now needs to get another electron from somewhere to regain stability. Antioxidants can supply that electron, but if they are in short supply the atom will rip an electron from somewhere else.
Redox concerns the transfer of electrons from one molecule to another. During that transfer one compound gains an election (is reduced) while the other loses an electron (is oxidized). (I don’t know why the compound which gains an electron is “reduced“, although it may be because electrons carry a negative charge.)
This movement of a charged electron from one molecule to another is a touchy situation. If that process doesn’t proceed correctly, then free radicals – imbalanced charged atoms or molecules – with unpaired electrons in their outer shells result. In their frenzy to regain their electrical balance, they can rip holes in the lipid coverings of cells, damage proteins and even alter our DNA.
Free radicals are only dangerous, though, when there are too many of them. Our body actually uses free radicals in a number of ways. Our immune cells use them to drill holes into pathogen-infested cells and kill them. They also play a crucial role in many cell signaling processes, and play a key role in aerobic energy production (where NAD+ is reduced to NADH and oxidizing NADH is oxidized to NAD+). In fact, you could think of the mitochondria as a massive free radical generation zone.
Our bodies use antioxidants like glutathione, superoxide dismutase, Vitamin C, Vitamin E, etc. to keep the oxidants (hydroxyl, hydrogen peroxide, superoxide) and nitrogen species (peroxynitrite) our body inevitably produces in check.
Insufficient antioxidant levels, however, can result in a free radical cascade as the damage they cause lets loose more free radicals – which cause more damage – producing more free radicals and on and on. High levels of free radicals have been implicated in many diseases including cancer, stroke, heart attack, diabetes, and ME/CFS.
The SARS-CoV-2 virus disrupts the mitochondria, potentially depleting energy and increasing oxidative stress.
Given that the immune cells generate oxidative stress to kill pathogens, it’s no surprise that oxidative stress would be increased in acute COVID infections. SARS-CoV-2, though, comes with a special twist: when it enters the ACE-2 receptor by elevating Ang II levels, it produces mitochondrial dysfunction and accumulations of the superoxide radical and other oxygen and nitrogen species.
With over a dozen studies finding a redox imbalance in ME/CFS, high levels of oxidative/nitrosative stress may be the most consistent finding in all of ME/CFS. Interestingly, levels of oxidative/nitrosative stress rise to particularly high peaks after exercise in ME/CFS. Metabolomic studies indirectly suggest the presence of high levels of oxidative stress.
A variety of studies have also found high levels of pro-oxidants – chemicals that either directly produce oxidative stress or impair antioxidant levels – in both ME/CFS and COVID. They include ferritin, free iron, homocysteine, neutrophil extracellular traps, altered levels of nitric oxide and hydrogen sulfide (in COVID), and altered tryptophan metabolism.
We can add an absence of the protective compounds – the antioxidants – which keep these oxidants in check to the list. Low levels of Vitamin E, ascorbic acid, and most importantly glutathione have been found in ME/CFS. Either people with ME/CFS are not producing enough antioxidants or their antioxidant system is getting overwhelmed by protein, lipid, and DNA-damaging free radicals.
The most important player in the redox issue in ME/CFS and COVID may be the mitochondria. The virus directly interferes with the mitochondria to produce an mtDNA-induced inflammasome, which suppresses both our innate and adaptive immunity. SARS-CoV-2 infection of white blood cells results in something which appears very like what we see in ME/CFS – increased rates of glycolysis, high rates of reactive species and lactate levels, and blunted energy production. One study found increasing antioxidant levels actually decreased viral load in COVID-19 patients.
Numerous problems with energy production have been found in ME/CFS. A hypometabolic state characterized by increased glycolysis, reduced ATP production, high blood lactate levels after exercise, elevated lactate levels in the brain, and elevated serum LDH levels (among others) appears to be present.
While it’s not clear what’s causing the mitochondrial problems in ME/CFS, the NLRP3 inflammasome protein complex appears to play a role in the inflammation found in the disease. This intracellular inflammasome reacts to signals (microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs)) generated by the cell to warn of a pathogen attack or damage to the cell. Increased levels of mitochondria-produced free radicals are one of the “danger signals” that activate the NLRP3 inflammasome. Given the mitochondrial issues in both COVID and ME/CFS, it’s likely this inflammasome has been activated.
The authors propose that an infection-triggered mitochondrial breakdown in long COVID and ME/CFS disrupts the redox balance, and produces massive levels of free radicals, which then feed an inflammatory process that impacts the blood vessels, in particular, but also the brain, the muscles, etc.
Over time, in diseases like ME/CFS with reduced antioxidant levels, a positive feedback loop is established: the high levels of mitochondrial-produced reactive oxygen species (oxygen-based free radicals) damage the endothelial cells lining the blood vessels – producing inflammation – which produces more free radicals – which causes more damage, etc. Essentially a fire gets lit that never gets put out.
While the authors focus on redox, they end up in a quite similar place as other hypotheses: inflammation, and mitochondria and blood vessel damage. Those endpoints are coming up again and again.
The redox issue, of course, is not limited to ME/CFS. Paul’s been focusing on redox issues in central nervous system diseases including Alzheimer’s Disease and Huntington’s disease, and has written broad overviews of the subject. Progress with repairing the redox imbalances in those diseases could certainly lead to progress in ME/CFS and allied disorders.
Treatment
If ME/CFS and long COVID (PASC) are caused by a redox or hydrogen sulfide imbalance that has plunged patients into a kind of hypometabolic state, what is the remedy? Some studies suggest that bucking up the antioxidant systems of COVID patients may help and a large number of redox-boosting agents (glutathione (and glutathione donors), N-acetyl cysteine, sulforaphane, ubiquinol, nicotinamide, melatonin, selenium, vitamin C, vitamin D, vitamin E, melatonin plus pentoxifylline, disulfiram, ebselen, and corticosteroids) are available (including a new one – cysteamine – which Paul recently championed).
Yet, as the authors point out, redox-altering therapies have never resulted in “dramatic improvements” even in conditions with redox imbalances. Given the role redox reactions play in health and disease, the inability to dramatically move the needle on health has undoubtedly puzzled many.
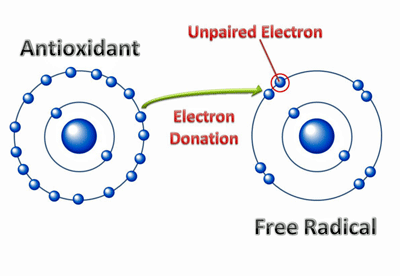
How antioxidants neutralize free radicals. Studies indicate antioxidant levels are low in ME/CFS.
The authors refreshingly point out that no single antioxidant can singlehandedly restore a damaged redox system to health. The fact that the problems in these diseases tend to be bi-directional (i.e. one problem feeds on another and vice versa) suggests that finding the core problem may be an exercise in futility. Instead, whole pathways and systems need to be lifted up together.
The Gist
- A recent review paper, “Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome“, authored by Bindu Paul, Marian Lemle, Anthony Komaroff and Solomon Snyder, appears in the Proceedings of the National Academy of Sciences.
- Paul, who was recruited to the ME/CFS field by Marian Lemle, received a Ramsay Award grant from Solve M.E. to study hydrogen sulfide in ME/CFS.
- The paper proposes that an imbalanced redox state, characterized by high levels of free radicals, may be causing long COVID and ME/CFS.
- Free radicals occur when molecules have too many or too few electrons in their outer shells, leaving them unbalanced. Unless antioxidants – which rebalance the free radicals – are present, the free radicals will rip electrons from neighboring molecules, causing damage to lipids, proteins or DNA.
- High rates of free radicals (oxidative/nitrosative species) may be the most consistent finding in ME/CFS. Low levels of antioxidants and high levels of prooxidants (free radical enhancing compounds) have also been found. Hi,gh free radicals have also been found in COVID-19.
- The mitochondria are the greatest source of free radicals in the body. The SARS-CoV-2 virus is able to disrupt mitochondrial function via its disruption of the ACE-2 receptor and its upregulation of Ang II. A similar ACE-2/Ang II interaction may be underway in ME/CFS as well.
- The authors propose that an infection-triggered mitochondrial breakdown in long COVID and ME/CFS disrupts the redox balance, and produces massive levels of free radicals, which then feed an inflammatory process that impacts the blood vessels, in particular, but also the brain, the muscles, etc.
- Over time a positive feedback process can result consisting of free radical production which damages tissues – releasing more free radicals – causing more damage and so on.
- While high rates of free radical production are also present in central nervous system and cardiovascular diseases, antioxidant supplementation has only had limited effects. This may be because whole redox pathways need to be enhanced. One way to possibly do that involves using hydrogen sulfide donors. Hydrogen sulfide will be discussed in a future blog.
- While the redox hypothesis focuses on a different system than past long-COVID and/or ME/CFS hypothesis papers, it is, rather encouragingly, circling around some common themes: the ACE-2/Ang II interaction, inflammation, energy production problems involving the mitochondria, and the blood vessels.
- In an interview, Bindu Paul reported that while central nervous system diseases like Alzheimer’s are different from ME/CFS and long COVID, a similar redox situation appears to be present and that treatments targeting that redox situation could benefit all three diseases.
The authors end by focusing on the similarities between ME/CFS and long COVID (redox imbalance, systemic inflammation, neuroinflammation, and impaired ATP production). With perhaps equal numbers of long-COVID patients joining the up to 2 1/2 million people with ME/CFS in the U.S., they declared that it is “imperative that increased research be focused on both long COVID-19 and ME/CFS” and write:
“We suggest that the study of the connections between redox imbalance, inflammation, and energy metabolism in long COVID-19 and in ME/CFS may lead to improvements in both new diagnostics and therapies.”
Bindu Paul Interview
It seems to me that you are saying that redox imbalances can play a major role in disease, yet we’re pretty poor at addressing that imbalance in an effective way. The paper also states “up-regulating pathways that counteract multiple abnormalities and bolster antioxidant defense and balance may be more beneficial.” It appears that instead of targeting specific antioxidants, finding ways to upregulate whole pathways could potentially help. Can you say what those pathways are?
There are several pathways that are protective. As discussed in the study, one of them is the transsulfuration pathway as outlined in the publication. This pathway is responsible for generating cysteine, glutathione and hydrogen sulfide. Cysteine, in turn is utilized for the synthesis of a number of sulfur containing molecules.
A point to be noted is that excessive levels of antioxidants may counteract essential processes such as autophagy. So, caution should be exercised while using single antioxidants.
Bindu, you’ve published quite a paper on redox imbalances in Alzheimer’s and neurodegenerative diseases and have proposed that hydrogen sulfide donors might be helpful. ME/CFS is obviously not Alzheimer’s disease (AD), but do you believe that some of the underlying processes might be present in both diseases?
Yes, redox imbalance plays a central role in the pathogenesis of AD, ME/CFS and COVID, based on the scientific evidence. There are common pathways which are affected in both neurodegeneration and in COVID-19 and ME/CFS. Both familial and sporadic AD are characterized by accumulation of plaques and tangles, both of which affect redox balance, especially in the brain.
ME/CFS is usually triggered after an infection. Although the causes for developing ME/CFS and AD are very different, they end up with elevated oxidative damage. The combination of the mutations in key proteins (Tau, APP, Presenilin and others) in AD in conjunction with the oxidative damage can elicit disease-specific effects. Regardless of the actual cause of the disease, preventing oxidative and nitrosative damage can be beneficial.
Might some treatments that attempt to alter the redox state in Alzheimer’s disease help with ME/CFS and other central nervous system disorders?
That is certainly possible.
Why might people with ME/CFS be susceptible to a redox imbalance triggered by an infection? Bad mitochondria? Insufficient antioxidant production? Genetic issues?
All or either of the above. As we discuss in the article, there are bidirectional connections between oxidative stress, mitochondrial function and inflammation. Excessive inflammation can trigger oxidative damage and mitochondrial dysfunction, which in turn causes inflammation, forming several vicious cycles that feed into each other.
While Alzheimer’s and ME/CFS are different, both result in high levels of oxidative stress and both could benefit from treatments that reduce it. (Photo by Gerd Altman Pixabay)
Are there commercially available tests that can accurately assess the state of the redox system in ME/CFS?
There are tests to measure levels of individual antioxidants.
If you were planning a major effort to assess the role a redox imbalance plays in ME/CFS and long COVID what would it involve?
Measuring the status of redox signaling as a function of disease progression. There are of course multiple ways to study redox balance. It is important to classify patients in terms of severity of the disease. Patients exhibiting mild symptoms may have a different redox profile as compared to those with severe disease. When oxidative damage, inflammation and mitochondrial dysfunction crosses a certain threshold, that is when the disease progresses to the severe stage. Additional funding would greatly facilitate these studies.
Conclusion
This PNAS review paper presents another intriguing possibility for chronic fatigue syndrome (ME/CFS) and long COVID (PASC). The authors are not alone in believing that a redox imbalance plays a crucial role in COVID-19. Another recently published hypothesis paper proposed that a similar scenario:
“We here consider COVID-19 as a redox disease…An inflammation-driven “oxidative storm” alters the redox landscape, eliciting epithelial, endothelial, mitochondrial, metabolic, and immune dysfunction, and coagulopathy.”
Like Paul et. al. they believe the endothelium – the cells that line the blood vessels – which they call the “gatekeeper of vascular health” plays a critical role in COVID-19.
The paper circles around a bunch of common themes found in other ME/CFS hypotheses; inflammation, ACE-2/Ang II, mitochondrial, and energy production problems, possible blood vessel damage.
Hydrogen sulfide has the potential to improve redox pathways. A blog on H2S, ME/CFS and long COVID is coming up.
The main source of free radicals (oxidative and nitrosative species) in the body – the mitochondria – are where the problem starts. While studies have failed to consistently pinpoint a specific problem in the mitochondria in ME/CFS virtually all the ME/CFS mitochondrial studies have found that something’s gone awry. Oxidative stress studies have produced probably the most consistent results in the ME/CFS literature: it’s clear that free radical levels are high, antioxidant levels – particularly in the brain – are low, and as the authors note levels of prooxidants appear to be high.
With regards to COVID-19 numerous studies and papers have focused on redox issues and treatment approaches are being proposed and tried. While we don’t have data on mitochondrial problems in long COVID many studies have assessed the effects COVID-19 has on the mitochondria and new treatment approaches are being proposed as well.
The authors propose that a fire gets lit in these diseases that never gets put out. Redox imbalances have been found in central nervous system disorders, cardiovascular diseases, ME/CFS, long COVID, and others yet effective treatments have not yet been found. That could be changing with the introduction of hydrogen sulfide treatments, and it’s to hydrogen sulfide that an upcoming blog will focus on.
Health Rising is entirely Community Supported
Please Support Health Rising!






For years, at every visit, I would argue with Dr. Paul Cheney about the role of environmental toxins in the onset of ME/CFS. He maintained a virus came first and that made you more vulnerable to the effects of toxic exposures.
I, on the other hand, maintained that some people of genetically vulnerable to chemicals and environmental toxins that can weaken the immune system and open the door to reactivations of latent viruses, myoplasma, and pathogenic bacteria.
This study discusses redox toxicology of environmental chemicals causing oxidative stress.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7327986/
It would be interesting to see a study that examined environmental exposures and chemical body burdens in both ME/CFS and long Covid patients.
I think you were both right. Toxic exposures are still underappreciated in ME/CFS although I hope over time that as the findings in Gulf War Illness merge to some extent with ME/CFS and long COVID they will be more appreciated.
There can be differences – when I visited Dr. Rea in Dallas I saw people with toxic exposures pounding away at the bicycles (then ducking into the saunas). I couldn’t begin to do that – but that’s not so in GWI where they have similar exertion problems to ME/CFS.
Cheney, I think, would appreciate the recent findings suggesting that the coronavirus can smack the mitochondria hard – and possibly through the same channel that they get hit in ME/CFS.
One focus I hope the emphasis on the coronavirus will trigger in ME/CFS is a deeper look into ACE-2 and Ang II. Except for a few studies it’s virtually been ignored – and now here it is showing up in all these hypotheses!
Did you get results from Rea? I went for Mycoplasma but didn’t as their phlebotomists could not deal with my veins.
I did the sauna once a day without exercise for 6 weeks I think. It was pretty brutal but a couple of days later as I was driving home I felt great! It didn’t last that long but something had clearly happened.
One additional comment about my visits to Dr. Cheney. At every visit, he would dictate something about changes in my fingerprints. I never knew what he was referring to until I actually saw a picture of the fingerprint changes he was noting.
I used an ink pad at my office and did a fingerprint. I was shocked to see that my fingerprint looked just like his sample. Dr, Cheney found these fingerprint changes in 40% of his patients and over more than 35 years he saw thousands of ME/CFS patients from all over the world.
He attributed these changes to oxidative stress.
Finally, I understood why my laptop and cell phone fingerprint scanner just wouldn’t work.
I suggest that you try this for yourself. Just get an ink pad and do a fingerprint and compare it to the one in this blog.
https://nopostergirl.com/2011/02/27/the-fact-of-fingerprints/
Cheney was the first and foremost doctor to recommend enhancing glutathione – the bodies most important antioxidant – for ME/CFS.
Right on, Betty!
I got ME/CFS by having mono while living in an apartment with a toxic mold problem.
Now I’m trying to get well from Long COVID and hoping my current living space is reasonably non-toxic.
Mold! Another incredibly poorly studied field. I imagine at some point historians will look back and ask how researchers missed it. I wonder how many mold studies the NIH has funded. In fact, I wonder if mold shows up in ANY of their Program Announcements.
I’ve done weekly IV glut and bedtime NAC but, alas, my best results arrived in gaseous format. However, that piffled out due to my inability to maintain inputs. It’s treated like crime to try to maintain Ozone Infusion care protocol.,though totally legal many states. Some oher substances associated with like problems– I read of B6 variant glycation motifier that was pulled from nutrient sale due to commercial interes in development..
This was me too. I definitely had precursors for ME/CFS, but was living fairly normally. Then ended up in a moldy apartment, and the rest is history.
CLASSIC! Two pathogens (as I read an eternity ago) are a prime primer for weakening and partial disabling the immune system. I wish someone might study this phenomenon.
Mine was a combo of mold (and other unknown but strongly suspected toxins – found out afterwards a few people got ill sitting where I was) from my workplace and the flu vaccine, to which I reacted very strongly and strangely and which markedly started my downhill health trajectory.
And, it turns out although no-one tested me for years, I have chronic EBV.
This combo is in SO MANY of our stories on here (including the Lake Tahoe cohort for mold and so many of us whose illnesses started subsequent to vaccines), yet these seem rarely studied. Why is that? Something is amiss that these COMMON CAUSES get little to no attention.
And, I believe as per Lisa Petrison’s recent comments on here, sewer gas leaks (which it turns out are disturbingly common but notoriously difficult to diagnose and plumbers are loathe to examine or deal with) have also been a factor for me, and I wonder how many others?
Too much ME/CFS research comports with the old story of the Victorian looking for his lost key under the gaslamp instead of where he dropped it ‘because that’s where the light is’. Researchers looking in their own areas, instead of where ME/CFS patients histories (and epidemiology – does no-one do that anymore?) have identified likely root causes or aggravating factors – which remain ignored with a curious lack of interest all around.
We didn’t all just spontaneously become ill in huge numbers out of no-where due to genetics; this disease has exploded our ranks over definable time periods, and yet those environmental (or other) factors which correspond and may be related remain unexamined, with little curiosity about them.
How do you think all of the common antibodies found in people with ME/CFS, POTS, etc. play into all this?
Hopefully they play a lot into this as they too can impact the blood vessels! They’re certainly showing up in a number of hypotheses.
Interesting thanks Cort. Whenever I take lipsomal Gluthione I get a relapse with burning lactic acid type pain in muscles.
Good to hear that liposomal GSH helps with burning lactic pain in the muscles – as that has plagued me for decades. :). Any particular type you recommend?
From what I’ve read glutathione is the most important antioxidant in the body. Low glutathione levels in the ventricles of the brain have been documented several times by Shungu and he is now engaged in a NAC clinical trial for ME/CFS.
Seems like Linda is getting worse with glutathione, not better?
I also got worse on glutathione, an extreme headache. I suspect part of it is dose accuracy issues. Too much is as bad as too little even potentially toxic, when trying to get balance in a pathway and there is no reliable way to eatable what is required. Look forward to the hydrogen sulphide article. Given individual antioxidants aren’t effective.
According to dr Andy cutler ( safe mercury chelation protocol ) taking glutathione could be bad for you especially as an IV , as the form as a supplement is not able to be absorbed by the body , the best form to take it is precursors l glutamine and glycine which your body converts to glutathione
As always, really fascinating. Thank you.
I’m super curious about how pentoxifylline might help as part of the treatment. Did you learn about that?
Thank you for this excellent article! I feel that we’re getting closer and closer to possible solutions.
FYI, gut problems can also contribute significantly to redox imbalance!
https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(20)30157-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS147149142030157X%3Fshowall%3Dtrue#secst0060
Fascinating. She too is looking deeper than the ordinary antioxidants. I like the idea of a ‘reactive species interactome” (RSI)
Hopefully, this field is coming of age. This would be quite a study
“To make further progress, studies are needed that integrate redox signaling readouts with other multi-omics datasets, including genomics, transcriptomics, metagenomics, metabolomics, and proteomics, accompanied by detailed phenotypic patient stratification. “
This is a very good summary, thank you! I wonder though if we shouldn´t give some attention to the “old” specifity question that so much plagues ME/CFS research: are the pathological events observed (here: redox imbalance) SPECIFIC to ME/CFS? Or are they to be seen in many other inflammatory diseases?
After all, mitochondrial dysfunction and inflammatory processes have been shown to be closely interlinked. Inflammatory activity in general, via proinflammatory cytokines, can cause mitochondrial fragmentation and dysfunction. Also, cytokines like TNF alpha can derange mitochondrial energy production and induce mitochondria to produce ROS thereby amplifying the inflammatory process. So clearly, mitochondrial dysfunction may be both cause and effect of inflammatory processes: inflammation may impede mitochondrial function, but mitochondrial dysfunction may also promote inflammation.
So in this sense a profound redox imbalance should be expected in ME/CFS – but it may not be much different than what you may see in other inflammatory disorders. And indeed, as the study points out, redox issues are also part of neurodegenerative (and other) inflammatory diseases. So the understanding of the redox feedback loops may add to the understanding of chronic inflammatory disorders in general, but does it add to the understanding of ME/CFS? (Which is not to say that this approach may not be helpful in finding new therapies!! It is not my intention to talk down Paul´s work!!)
If this holds up some aspects of this must be specific to ME/CFS: type of redox imbalance that’s present, how it’s triggered, or where it occurs perhaps. Otherwise, yes it appears that redox imbalances are found in a number of diseases and they are quite bidirectional – making it hard to tell what started what. I don’t think we will know about the specificity (or not) of the problem until we get more detailed research.
Even the redox imbalance in ME/CFS is similar to that found in other diseases what excites me – and what appears to interest you as well – is the idea of new approaches to resolving what appears to be a fundamental problem; i.e. if it’s not the answer hopefully it could still help.
Are they having good results with NAC Cort? Have been thinking about trying it but always wary to start new things.
The initial results were really good but only in patients who showed they had reduced glutathione levels in the brain. I don’t know what percentage of patients levels were low in but it I believe it was a pretty high percentage.
Hey Cort. This is really interesting I’ve been meaning to try NAC myself but not sure about dosage or how long to use it for. Any ideas? Thank you sir.
I don’t but I am going to try and find out. A blog on NAC is coming up.
50 Years of Cfs and we still fund studies that tell us to take vitamin C and hope for the best lol. Yea, we are doomed if even the small money we have is wasted on useless stuff like this.
The NAC study is a big NIH-funded one. It was funded after a smaller study found that NAC not only improved symptoms but actually increased GSH levels in the brain.
The FDA wants to enforce the ban the sale of NAC as a supplement
https://www.naturalproductsinsider.com/regulatory/us-senator-npa-press-fda-nac-supplements
Christopher,
Perhaps you’ve missed the large sections of Cort’s forums — and those of his old forums at Phoenix Rising, where by far the majority of patients who are improving, are those addressing mitochondrial dysfunction, methylation, gut issues, etc., with individualized SUPPLEMENTS.
There will never be ‘a cure’ — one single cure — for this disease, as we all got sick in (many) different ways. I hope you’re not waiting for that ‘magic pill’!
Cort,
Thank you for listing about this. It’s the exact same problem I’ve gone to 4 mito specialists about. Unfortunately, they didn’t know how to help me as I don’t have a genetic mito disease, only acquired mito dysfunction.
I have increased my energy by taking a balance of antioxidants from Lester Packer’s antioxidant network – one time I had to have a CT scan and researched protecting myself from radiation effects – the answer was to take antioxidants… So, I doubled my normal amounts of ALA, glutathione, A, C, E, and CoQ10, and went for a walk. At the end of my walk, a car was coming, and I was able to RUN across the street, something I usually can’t do!
So, taking them.in combination daily had been helpful. I also take melatonin, curcumin, sulforophane and a pomegranate extract, as well as NT Factor, which repairs mitochondrial membranes damaged by oxidative stress and C, folate and B12 to reduce nitrosative stress (Martin Pall’s protocol). Thomas Seyfried’s mitochondrial correction protocol had been helpful, too.
I’ve been able to reduce 8OHDG a market of mitochondrial damage with all of this, yet I still have high lipid peroxides, which damage cell membranes. I’d like to hear more about how we can manage/treat these problems as the ME/CFS Clinician’s Coalition seems to ignore this issue.
Did you write a column on hydrogen water, Cort? Not sure if it is comparable as hydrogen sulfide described in this research. And here is a DYI for making this water; https://www.youtube.com/watch?v=mJ1DeiD5LTo
Please correct me if I am wrong, but I remember reading glutathione supplements don’t hold up well in the stomach and it may be better to take the precursor, N-Acetyl L-Cysteine.
Same with NAD(H) and that’s why the supplements are sublingual.
Stanford news; apparently they are reshuffling their CFS clinic and now call it PACS ME/CFS which I assume is where they will be addressing long haul Covid. Long time PA Sangeetha Kandan has departed and there are now new people there. Bonilla is still the main M.D. and is focused on infective causes and neuroinflammation. I got bumped from my upcoming appointment and am now on a waiting list…I’m sure they are now busier than ever.
I, on the other hand, have discovered that if I drop my levothyroxine (T4)(I’m symptomatic euthyroid with high autoantibodies–makes sense, huh?) and just take a small amount of liothyronine (T3), my energy is improved somewhat. Seems as if some are poor converters and I read a new paper that says taking both T4 and T3 together actually blunts T3 activity somewhat. Haven’t told my doctor yet and fear she will freak out!
Am tooting away on my didgeridoo but probably haven’t been consistent enough to show any meaningful changes.
And Betty, regarding fingerprint changes, those of us with EDS frequently don’t have much fingerprint ridges regardless of whether we have a ME/CFS diagnosis or not. Even so, many of us suffer from pain and fatigue… I have to laugh because I cannot open those produce plastic bags in the grocery store, no matter how hard I try!
Poco a poco regaining some energy. Haven’t tried the Colchicine yet…
Nancy!
https://www.healthrising.org/blog/2019/03/07/thyroid-t3-chronic-fatigue-fibromyalgia-recovery-stories/
– read through the comments too, to learn about Dr. Blanchard’s microdosing approach.
Also:
https://www.researchgate.net/publication/348841282_Hypothesis_Mechanisms_That_Prevent_Recovery_in_Prolonged_ICU_Patients_Also_Underlie_Myalgic_EncephalomyelitisChronic_Fatigue_Syndrome_MECFS
https://www.researchgate.net/publication/351373802_Theory_Treatments_for_Prolonged_ICU_Patients_May_Provide_New_Therapeutic_Avenues_for_Myalgic_EncephalomyelitisChronic_Fatigue_Syndrome_MECFS
You can read Dr. Roda Barnes and Ray Peat.
You can learn to track your thyroid function by measuring your heart rate and temperature.
Fire your endocrinologist if they are not on board with this approach!
Meirav!
Thank you for posting those helpful links on thyroid! I consider them ‘armament’ for the ensuing argument I will have with my endocrinologist!
Tracking the rhythms of the body is so much more elegant than using a single medication ‘hammer’ to nudge the thyroid system back into sync. Wish it was easier to find endocrinologists willing to think outside of the box.
Nancy,
How are you getting the T3 if your doctor isn’t prescribing it? I’m in the same boat as you (euthyroid symptoms, thyroid antibodies, very high reverse thyroid hormone) and would love to try this, but my doctor won’t venture beyond the narrow confines of his training (had to bully him to even measure for reverse thyroid ‘Why would we do that? There’s nothing that can be done about it?’).
Anne,
Maybe try some Thrytrophin PMG made by Standard Process. It has T1,T2, and T3 in it.
This sounds a lot like Dr. Martin Pall’s NO/ONOO nitric oxide cycle hypothesis, which he described in his 2007 book. I managed –just– to get through the book back then, but my brain no longer works that well, so I can’t confirm.
It also reminds me of Rich Van Konynenburg’s papers on the methylation cycle block hypothesis.
Those cycles are too complex for me to follow these days, but I would suggest that anyone researching redox in ME and Long Covid should look them up.
I’m glad that the research is being done, because they couldn’t get funding for it back then. It’s a shame, because we might be further along in our understanding if they had.
I thought of both of them too. We’re going to have a blog coming up concerning a clinical trial in ME/CFS that’s going straight at glutathione. Too bad Rich is not around. He was a true gentlemen -and Bindu Paul is very aware of nitrosative species like ONOO-. How interesting that we got these efforts going in this direction so long ago.
Did anyone stop to wonder if these excessive free radicals and oxidative stress are there for a REASON? Maybe that’s the body’s intelligent way of trying to fix itself and (too many) antioxidants are keeping people sick?
It’s worth noting that Dr. Paul said: “A point to be noted is that excessive levels of antioxidants may counteract ESSENTIAL processes such as autophagy. So, caution should be exercised while using single antioxidants.”
“There is mounting consensus that ACE inhibitors may be a primary driver of the severe symptoms. The concerns have been raised in the Lancet8 and most recently in Medscape”
I take Lisinopril an Ace inhibitor. could it be?
Another one with no finger prints, just try to open one of those plastic bags in the vegetable section warring a mask, I’m sorry I lifted the mask and licked my finger. Please don’t tell or they may put me in jail. We need to put a stop to the Nazis.
A tip for those of us with altered fingerprints who can’t open plastic bags in the vegetable aisle (huh – hadn’t realized before now that that was a thing, but yep I have it too): instead of licking our fingers (which is unsanitary) find the vegetables they periodically spray with water and wet your fingers there. Or if they don’t spray their vegetables with water, go to the freezer section and touch the inside of the freezer to generate condensation – your warmth will create moisture. Failing that put a bag of frozen veggies in your cart; thawing will create enough condensation to wet your fingers.
a ha ha! is this really a thing many have?
I thought it had to do with bendy fingers….
Cort,
Would you ever consider moving ‘The Gist’ up closer to the top of your article? Or perhaps putting a big ‘Brainfogged? See The Gist below’ button with a hyperlink at the top of the page?
For some reason I never remember it’s there, and usually only notice it after the time my brain is too tired to read on, so I miss big parts of your articles.
<>
Cort, it’s an historical thing, going back to the beginnings of chemistry. Originally, “oxidation” meant gaining oxygen, as in burning wood, while “reduction” meant losing oxygen as when heating metal ores to extract metals. The metal was then lighter than the ore, having lost oxygen, hence the ore was “reduced”. Since then the understanding of the process has been generalized to reactions that don’t involve oxygen and to incorporate atomic theory but the chemical language stuck. See here:
https://en.wikipedia.org/wiki/Redox#Etymology
Interesting – so HBOT, or Airnergy could be a work around as they both help to reduce free radicals along with a fecal transplant, healthy eating such as the Keto and the right vitamin combination and bio identical HRT .. all very expensive and the NHS will not fund –
Keto definitely isn’t ‘healthy eating’. In fact it’s probably the worst thing for people with ME/CFS who most often can only tolerate carbohydrates…
I agree w/you though on HBOT. 🙂