Led by Cheng Guo (lead author) and Brent L. Williams (senior author) this big NIH-funded study from Ian Lipkin’s Columbia team at the “Center for Solutions for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (CfS for ME/CFS)” set out to fix some of the problems found in prior gut studies. The study “Deficient butyrate-producing capacity in the gut microbiome of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients is associated with fatigue symptoms” was bigger (n=200), more rigorous, and used better bacterial sequencing techniques than past studies.
A lactobacillus gut bacteria (Dr.-Horst-Neve-Max-Rubner-Institut-CC-BY-SA-3.gif). This study found a reduced diversity of gut bacteria in ME/CFS.
Ian Lipkin’s team involved seemingly everyone possible (Mady Hornig, Anthony Komaroff, Susan Levine, Lucinda Bateman, Suzanne Vernon, Nancy Klimas, Jose Montoya, Dan Peterson) in this big NIH-funded study. The study was not just big – it was long as well. Study participants provided stool samples 4x’s over a year and completed a multidimensional fatigue inventory each time to boot.
Plus, following past studies from this group – the study assessed the effect irritable bowel syndrome – a common but certainly not universal comorbid condition found in ME/CFS – has on gut flora. Finally, as it did in a past study, this new study added metabolomics to the mix as well.
The study also used a technique that appears to be new to ME/CFS gut studies called “shotgun metagenomics sequencing” which allows researchers to comprehensively sample all the genes in all the organisms found in a complex environment. This technique allows researchers to detect bacterial species that other methods are likely to miss.
This study provides us clearly our best look yet at the gut bacteria in chronic fatigue syndrome (ME/CFS).
The Irritable Bowel Syndrome (IBS) Connection
“Both our current and prior fecal shotgun metagenomic study support the notion that IBS morbidity must be carefully considered in future ME/CFS microbiome studies”. The authors
Irritable bowel syndrome (IBS) is characterized by symptoms like abdominal pain, cramping or bloating usually associated with a bowel movement, changes in the appearance of bowel movements (ranging from hard-packed feces to diarrhea), and changes in how often you’re having a bowel movement. About a third of the ME/CFS patients in the study had IBS.
A Less Diverse Ecosystem
Having IBS made a real difference. Specifically, the differences in the alpha diversity of the gut bacteria largely reflected whether one had IBS or not. Alpha diversity is a term borrowed from ecology that measures the richness or diversity of complex ecosystems (like a forest or a gut). Like a forest, the higher the species richness or alpha diversity of the bacteria in the gut the more resilient, healthy, and productive the gut should be
The bacteria in our guts form an ecosystem. This study suggests that some ME/CFS patients’ gut ecosystems are less diverse and less resilient (“Wild Garden of the gut bacteria” – from Wikimedia Commons”)
Alpha diversity isn’t the only measure of gut health but it is an important one. One Ph.D. student reported that “High gut alpha diversity has been linked to a healthy state in so many studies that it has become common knowledge in microbiome circles.”
Having IBS had a dramatic influence on alpha diversity with only the ME/CFS +IBS having a reduced alpha diversity compared to healthy controls.
Beta diversity can also be used to assess gut health. The beta diversity test compared the difference between the microbiome species found in the presumably healthier guts of the healthy controls with the ME/CFS patients with and without IBS. In contrast to the alpha diversity finding, both ME/CFS cohorts (those with and without IBS), had altered beta diversity; i.e. whether they had IBS or not, the flora in the ME/CFS patients’ guts was markedly different than the flora found in the healthy controls’ guts.
“A Potential Biosensor of Human Health” Reduced
The study found that the relative abundance of two species (E. rectale and Faecalibacterium prausnitzii ) and six genera (Eubacterium, Faecalibacterium, Dorea, Roseburia, Gemmiger ) differed in relative abundance in ME/CFS compared to healthy control subjects.
F. prausnitizi stood out because it’s been found reduced in at least one fibromyalgia and two ME/CFS studies. This odd bacterium, which does not produce spores, and moves around very little, makes up a full 5% of the bacteria found in our guts. Through its fermentation of dietary fiber, F. prausnitizi produces butyrate and other short-chain fatty acids, and an important anti-inflammatory product. It’s been called “a potential biosensor of human health”.
The authors noted that low fecal F. prausnitzii levels have been found in inflammatory bowel disease (IBD), IBS, celiac disease, colorectal cancer, obesity, in people with COVID, and in long COVID. The Flemish gut project found that Faecalibacterium and Coprococcus bacteria were associated with higher quality of life scores, and reduced levels of F prausnitizii have been associated with fatigue in inflammatory bowel disease.
Reduced Butyrate Levels – A Seminal Gut Finding in ME/CFS
Fatigue was certainly the byword in this study as lower butyrate levels were associated with more fatigue and both general and/or physical fatigue were particularly associated with reduced levels of F. prausnitz, Coprococcus, and a few other bacteria.
Butyrate’s becoming a pretty big deal in ME/CFS gut studies. It’s the primary energy source for the endothelial cells lining the gut – an interesting finding given all the focus on the endothelial cells lining the blood vessels in ME/CFS and long COVID. Given the strong possibility that leaky gut is present in ME/CFS, it’s intriguing that butyrate protects the gut lining. Its support of regulatory T-cells and its anti-inflammatory activity helps keep the immune system in check as well.
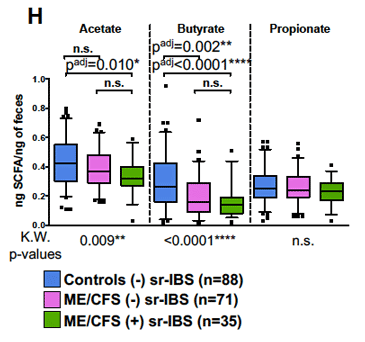
Dramatically reduced butyrate levels (middle column) in ME/CFS patients (with IBS – green; without IBS – violet) compared to healthy controls.
Reduced butyrate levels in ME/CFS then, could result in an inflammatory state in the gut and a weakened gut barrier to boot; which is perhaps just the right combination to spark an inflammatory response that ranges from the gut all the way to the brain.
The deeper the researchers dug, the more solid the low butyrate finding became. A metagenomic analysis snagged one source of the butyrate problem when it found that people with ME/CFS tended to be deficient in one of the two genes (the “but” gene) used to produce butyrate using the acetyl-CoA pathway gene. A functional analysis then indicated that the bacteria using that gene were the same bacteria that people with ME/CFS were missing.
The butyrate connection got even stronger when the authors found reduced levels of a substance called acetate. Because butyrate-producing bacteria need acetate to produce butyrate, the acetate deficiency fit the bill.
The Gist
- Ian Lipkin’s group presented the findings of the largest gut study ever done in ME/CFS.
- The study found that having ME/CFS and irritable bowel syndrome – reduced the “alpha diversity” or richness of their gut flora – resulting presumably in less productivity and resilience. Just having ME/CFS, on the other hand, did not.
- As several other studies have found the bacteria that produce butyrate – an anti-inflammatory that provides essential energy resources to the endothelial cells linking the gut wall – were deficient in ME/CFS. Low levels of butyrate were associated with greater fatigue as well.
- With this study, three different methodological approaches have identified low butyrate levels in ME/CFS patients’ guts – making it one of the more solid findings in all of ME/CFS.
- Low levels of butyrate could result in inflammation, weakened gut linings, and leaky gut – potentially producing systemic inflammation that reaches all the way to the central nervous system.
- This also makes this study the third to find reductions of a specific butyrate producer called F. prausnitizi. Low levels of F. prausnitizi , which has been called a potential biosensor of health, have been found in a number of chronic diseases including IBS, IBD, and fibromyalgia.
- Because acetate is needed to produce butyrate, the reduced acetate levels found made sense as well.
- What was causing the higher-than-normal levels of bacteria in ME/CFS patients’ feces was unclear.
- Recent studies which have shown that reduced activity levels are associated with reduced levels of butyrate bacteria suggest one reason why butyrate-producing bacteria may be low in ME/CFS.
- People with ME/CFS could be caught in a kind of low butyrate “loop” where low butyrate levels spark inflammation, which produces fatigue thus reducing activity levels, reducing butyrate levels further, etc.
- GIven that, it’s possible that increasing gut butyrate levels in ME/CFS could reduce inflammation, reduce fatigue, increase activity levels, etc.
- An upcoming blog will focus on increasing butyrate levels.
The fact that three different techniques (qPCR, metabolomics, metagenomics) have now found low butyrate-producing bacteria in ME/CFS makes the butyrate gut finding possibly one of the most solid findings in ME/CFS.
The fact that more people with ME/CFS than healthy controls (10%-2%) were taking supplements (prebiotic fiber) which should have enhanced their levels of butyrate-producing bacteria present only increased the mystery about the low butyrate levels studies have consistently found in ME/CFS.
Bacteria Packed Feces
Another interesting finding concerned the fact that the feces of the ME/CFS patients were packed with more bacteria than normal. The authors discarded antibiotic use, acute malnutrition, and inflammatory bowel diseases as possible causes and suggested several possibilities: low levels of FODMAPs (fermentable oligo-, di-,539 mono-saccharides, and polyols) foods, small intestinal bacterial overgrowth (SIBO), greater bacterial “washout” or the loss of bacteria that adhere to the mucosal walls.
FODMAPS diets can be helpful in IBS and SIBO appears to be common in ME/CFS. (The idea of malnutrition was intriguing – at least to this layman’s mind – given that the metabolomic findings in ME/CFS mirror those found in starvation and the possibility that the cells of ME/CFS patients may be starving in the midst of plenty. Could people with ME/CFS also not be utilizing all the nutrients in their food?)
The Activity Question
We don’t know why the butyrate-producing bacteria are so low in ME/CFS but low levels of physical activity may come into play. Recent studies (mostly done on rodents) indicate that physical exercise stimulates the same butyrate-producing bacteria that are deficient in ME/CFS.
It’s not clear why this is so but numerous possibilities (reduced gut motility, reduced blood flows to the gut, circulation of bile acids, etc.) exist. The association this study found between reduced butyrate levels and fatigue could, then, be caused by the lower activity levels that more fatigued people with ME/CFS engage in. (On the other hand, low butyrate levels are found in many diseases (inflammatory bowel disease (IBD), IBS, celiac disease, colorectal cancer, obesity) in people with COVID which do not feature the extreme activity limitations found in ME/CFS.) Clearly, we need gut studies that also assess activity levels to address this issue.
It’s clear to me personally, though, that low activity levels are not responsible for all the gut perturbations in ME/CFS as I am not deconditioned (and have the Oura stats to prove it), yet I still intermittently suffer from irritable bowel syndrome.
In a sense, the activity question doesn’t matter. Given that the guts of people with ME/CFS and perhaps fibromyalgia and long-COVID patients have low butyrate levels which could result in gut inflammation, leaky gut problems, and perhaps even systemic and neuroinflammation – the question is what to do about them?
It’s possible that people with these diseases are caught in a kind of low butyrate loop: their lowered activity levels (or something else) resulted in reduced levels of butyrate-producing bacteria – which then produced inflammation and leaky gut (particularly as a result of exercise), which resulted in more fatigue and less exercise, which further reduced their butyrate levels, and on and on.
One way out of this loop might be to increase butyrate levels, thus reducing the inflammation and leaky gut (particularly after exercise) allowing people with ME/CFS to be more active, resulting in more butyrate production, higher activity levels, and on and on.
- Coming up – increasing acetate and butyrate levels in the gut for better health






Don’t want to ruin your upcoming post, Cort, but I just read that eating nuts can increase butyrate-producing bacteria. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7468923/
Don’t know if that will work for me/cfs folks.
🙂
I have been taking probiotics for few months now resulting in reduced inflammation and muscle pain but still fatigued and stressed specifically at mornings.
I was wondering if there is any specific probiotic I should take, I keep reading about different probiotics that don’t seem to be in any of the products that I see in the pharmacy!
Any advice much appreciated.
Yes, soil based probiotics work best, along with pre-biotics and immunoglobulins and amino acids to heal the gut lining. The best triple powerhouse is Megasporebiotic with Mega-Pre and MegoMucosa along with AmazingGreens powders and glutamine and collagen powder mixed into a smoothie every day. The butyrate liquid can be added to it too, maybe toss some kidney or garbanzo beans in there since the fruit will mask the taste.
Liquid butyrate can also be taken daily but the best thing for increasing butyrate are greens and BEANS and proteins.
Getting a GI Map test or the BiomeFx test can tell you if you have low butyrate or not. Every human with an autoimmune disease has low butyrate so makes sense that those with CFS/ME do too.
Functional Medicine providers have known all of this for years!
Sodium butyrate. Amazon carries it. Keep taking the other pre, pro and post-biotics, as well. But first, start the protocol w/ two or three days of Ivermectin before starting your sodium butyrate and other microbiome supplements. Ivermectin cleans out the bad bacteria and allows you to “reset” with the good, fresh, healthy bacterium and enzymes.
Not well
This is fascinating and makes sense. I completely cured my sons seizures, severe epilepsy LGS diagnosis, by switching to the low glutamate diet and removing all triggers Onfi, Keppra, Trazadone, Miralax, and Epidiolex, and baclofen.
Dr. Kathleen Holt out of American University has published studies showing huge improvements made for fibromyalgia patients and gulf war illness patients. See link and search for videos she has made talking about the benefits. https://www.american.edu/cas/health/nutrneurolab/studies.cfm
I did the diet for a week and by day 3 my fibro pain was melting away. It is just hard to stay on when tired and can’t cook and I have a sweet tooth.
I wonder if Kathleen and Columbia could connect, bet there is a lot of overlap with their research.
Kathleen is wrapping up a pediatric epilepsy study soon that was showing promising results with the Low Glutamate for pediatric epilepsy patients.
It all starts in the gut.
Can increasing butyrate or butyrate producing bacteria cure or ameliorate Chronic Fatigue? That’s what I want to know.
And it shouldn’t be hard to find out. Just give people with CF, who also have low butyrate, a supply of the substance, and/or of the bacteria, and compare them with placebo fed people.
We already know there is an ASSOCIATION. Now let’s discover the direction of the CAUSALITY.
I will be happy to volunteer for the Clinical Trial.
I have been Gluten free , Soy free, and dairy free as much as possible for 3 years. Definitely can tell a difference. You do what you have to do to feel better. I also take a Good Probiotic 2 times a day. AM and PM. One is Klaire Labs Pro 5. The other is Master Supplements Theralac. Also Pure Encapsulations C0Q10 120 mg 2 times daily. I have not had Red meat in 4-5 years. Some Chicken with no gravy or crap Baked. These are things you can do for your self. But you do have to stay with it. I do not drink or smoke , no cokes , no orange juice . But I do eat oranges.
Butyrate is MADE in the gut. You can’t supplement it effectively.
<>
FWIW, I’ve recently been hit hard with new IBS symptoms, after 35 years with CFSME. I was surprised when my gastroenterologist specifically prescribed “Align,” vs other. This is from label:
Supplement Facts
Serving Size: 1 Capsule
Servings Per Container: 63
Amount Per Capsule
Bifidobacterium longum subsp. longum 35624TH+
20 matt
† Contains one billion live probiotic cells / CF when manufactured and
provides effective level of ten million probiotic cells / FU until at least
the “best by” date.
†+ Daily Value (DV) not established.
Other ingredients:
Microcrystalline cellulose, hypromellose; Less than 2% of:
magnesium stearate, caramel (color)
Contains: Milk
DIST. BY: PROCTER & GAMBLE, CINCINNATI, OH 45202
I’ve had ME/CFS for 18 years (since 2003/2004). I tried sodium butyrate the other year and when I took around 4 to 6 capsules daily, the fatigue lifted, however, after a few months the butyrate started giving me heart palpitations so I had to stop. I should have reduced the amount to see if it helped at a lower dose. Since then I paid to have a stool sample tested and it showed I’m too low on bifidobacteria. Bifidobacteria have all kinds of benefits including producing butyrate. I also started with loose bowel movements in 1999 – unable to digest a carb heavy meal; and low and behold bifidobacteria digest carbs. In 2008-2012 I had a 4 year remission from CFS and loose stools using homemade kefir minimum 1 to 1 and a half cups daily, plus grain free, low carb diet and copious amounts of homemade carrot juice and almond milk. After 1 and a half years I got fed up of drinking kefir every day so packed it in. After 4 years I went back to eating grains, albeit gluten free grains. The loose stools returned and over the next 5 years my fatigue became progressively worse until by 2017 it became severe again, leaving me bed bound at times. The point is, I recovered on kefir so I suspect that CFS/ME for some people is connected to an imbalance of healthy gut bacteria. Though I don’t know why some people recover on antivirals, unless a healthy gut microbiome is also able to keep latent viruses in check? I tried the kefir diet again but became discouraged after a month as it didn’t work the second time round. I don’t think I gave it long enough and when you have severe brain fog it’s hard to remember your name, let alone which protocol to do next. Nonetheless we press on. An AIP diet (and/or low histamine? low lectin? high lysine-low arginine anti-viral diet?) along with restoring the gut microbiome is a possible way to go for some of us? With you all in the fight. xx
i have been on supplemental butyrate for a long time due to testing to low which i no longer test low. however recently i had a terrible time with my gut and not sleeping. alot of probiotics and supplemental saccromyces boulardi helped but it was a slow process and i like brand Renew Your Life. I had high bacterial levels and high SIGA + sibo and test results rec i take calcium d’glutatrate but my gut didn’t want it. I also use alot of healthy herbs that are prescribed. Anyhow finally able to eat more normal, my diet has been on the healthy side for yrs-no sugar, organic.. grass fed meat not too hi in fat… (veggies and salad & berries) which i couldn’t handle at all during this ordeal. It was a genova diagnostics 3 day test.
That’s interesting about the nuts! I eat a LOT of nuts, and they definitely have not cured my CFS/ME – but I will pay closer attention to how they make me feel with regular ingestion 🙂
Same here! If nuts were a solution, I wouldn’t be bed bound.
I heard most beneficial are almonds and pistachios.
However, I think the caveat is to have butyrate producing bacteria in the first place. That’s the hard part. It takes a long time to rehab the gut. >1 year.
There are butyrate supps that may help in the meantime whilst rehabilitating the gut.
Fascinating research. My adult son and I had Faecal Microbiota Transplants in December and have had an astonishing result. We’re building up slowly as we believe it’s an effective treatment, not a cure. I can now exercise without pain or fatigue, wake refreshed and alert. After activity we’re tired not fatigued. IBS, pain, migraines all improved. No brainfog but still have some cognitive disfunction.
I am absolutely in tune with this report, which confirms my intuitions and current practice, as a three decade ME/CFS victim of establishment gaslighting, who long since has resorted to cutting edge research and self-care.
I understand and have experienced that nuts do tend to have significant amounts of oxalate which may be irritant and also can block the absorption of nutrients.
If not consumed with great care, in moderate amounts they may cause purging and *must* be very well chewed ( to cream) in moderate amounts or powdered in a blender and added to smoothies.
That’s my experience, and in ME/CFS, one size does not fit all.
Some contend nuts must be soaked to remove irritating substances.
I eat Raw Almonds all the time. The only one that does not bother me. All Natural.
Hope there’s more to it than eating nuts 🤞
Well it is worth a try, but I eat a lot of nuts, almost daily, and that hasn’t worked for me. Unfortunately.
The article is a meta-analysis of 8 nut-gut studies. The authors conclude:
“The quality of the included trials was poor, with no studies experiencing low risk of bias across all domains. Overall, the strength of evidence from the meta-analyses is weak.”
No one wants to hear this-but “CUT OUT THE CRAP FOOD AND DRINKS”. YOU KNOW WHAT THEY ARE.!!!!!
I find that most types of nuts makes it worse. Cashews, for some reason, don’t.
Nuts increase Serotonin, good or bad depending, bad for me.
cashews are technically not a nut.
Mast cell activation with nuts tho…why is there always another snake rearing itps head wherever you turn with this disease
100
I wish I could eat nuts, I am unable to digest so many foods, and nuts is at the top of the list! I can digest proteins from fish & poultry. I am very limited with fruits & vegetables as well. I do best juicing both as I cannot digest them! Been fighting this for 20 plus years, but the past decade has been horrible, GI specialist after specialist, No help! Doing in depth GI & Bacteria testing to use “appropriate “ Probiotics again! This part of MEcfs really sucks! Thanks Cort for doing what you do to keep us all on the cutting edge of science and treatments!
Fascinating research. My adult son and I had Faecal Microbiota Transplants in December and have had an astonishing result. We’re building up slowly as we believe it’s an effective treatment, not a cure. I can now exercise without pain or fatigue, wake refreshed and alert. After activity we’re tired not fatigued. IBS, pain, migraines all improved. No brainfog but still have some cognitive disfunction.
These doctors are frauds.
Mold caused my plethora of digestive issues and infections and it wasn’t until I got out of exposure did my gut return to normal. To blame a person’s gut for why they are sick not on the external factors that could be causing the disarray in their microbiome is misleading.
Shame on them.
No a lot of people like me, their body can not tolerant nuts. 🙁
I cannot eat nuts either
all good and well, glad to read an ME/cfs study, but what about the faecal transplants? that help some, to a certain degree? and what about the findings before and after VO2 max test/exertion the microbiome changes in ME?
i find it so bedridden that eating “a few nuts”, will get me out of bed a little bit simplistic…
i even do not know what to think about the whole study in comparison to what the found before (before and after excercise, the guttransplants, etc)
cort, in an other post you put the link of the women who took so many pre and pro biotics, where you have written about. i got the text but all the links in it do not work anymore. is that normal? can you help? thanks!!!
There are various ways to increase butyrate levels in the gut – I didn’t know about nuts until now – but I promise there are more ways 🙂
Thanks for mentioning the exercise study which showed leaky gut after exercise. There’s been one fascinating study (https://pubmed.ncbi.nlm.nih.gov/26683192/ ) which showed this which could really use a major update – as it showed how exercise could be producing inflammation and fatigue and, of course, PEM.
Here’s the link to the woman who poured probiotics into her system and recovered – https://www.healthrising.org/blog/2016/01/07/probiotics-cure-my-chronic-fatigue-syndrome/ – Everybody should note that some people can actually do worse on probiotics.
The gut obviously counts in ME/CFS – we just don’t know how much.
thanks cort for the link, but you had allready given me that. but in the article, if i open it, there are some blue things to click on in your/her text. and it simply does not work. how can that be? you have written it. now i tried with the word “blog” in the text and a “crazy” site showed up. normally i would think it would still be a “link” to the original text that would be mentioned. does it disapear after x years?
yes, the gut is important in ME/cfs as you write. but how…
I saw once a webinar on gutmicrobiota and wich diet was good for wich person, it was really personalised. it was crazy what you saw.
maybe whole our gut problems are also personalised medicine because we are all so different.
I do not know if this study is from the centers of excellence where, i really give it him, ian lipkin was the only one who stood up and several times said with that kind of money, we will not get verry far. all the rest was quite.
and with the litlle money for ME/cfs, what is there still all to research on the gut? where no money is for?
but thank you for a piece of hope i certainly need, like many of us do.
Hi Konjin, I believe she wrote the blog and am not sure where those links should go.
This was, I believe (but am not sure), from Lipkin’s NIH-funded ME/CFS research center.
Some people may appear to do worse on probiotics due to injudicious use and the Herxheimer reaction. q.v.
Festina lente!
My baseline is dependent upondaily probiotic intake (have been taking for 3 years now). I’d been informed this is due to the immunomodulating effects of them I e they suppress over-active immune response. If I miss more than 3 days taking them I get very ill.
I’ve implemented Carol’s protocol and it’s been brilliant for me. But I get to a point where more probiotics don’t seem to do anything. I’m constantly stuck in a bell curve of remission and relapse. Although this only occurs after vaccinations currently. I’ve felt pretty great taking solarray 24 strain and Dr mercolas probiotics. I’ve also introduced fos, pysllium husk, garlic extract and egcg (green tea).
The thing is I now suffer from neutropenia and if my calculations are correct I’m due to become severely neutropenic in about 3 months. ME doesn’t just disappear when you find something that works – it can in fact make you feel worse in other ways. It’s an extremely complex disease.
Cort I also found it very interesting that you cited patients were already taking fibre but they had very low levels of the key bacteria. If so none of us would be able to rectify the missing bacteria with say biomesight suggestions because something else is causing it.
As ever I get a bit tired of seeing these studies that prove what has sometimes already been proven. We need to know why the bacteria are in such a bad way and why we can’t fix it by taking more fibre and more natural extracts that are meant to help feed the right bacteria. If it works for healthy controls why doesn’t it work for us?
Konijn,
Apologies for the long reply. Hopefully, you’re up for it.
I am always reluctant to give any advice about ME/CFS symptom management. People are very sick and I am not a doctor, just another ME/CFS patient (acute onset, post Ross River virus). I would never want to make anyone worse, yet alone someone who is bedridden. However, I think I’m in a reasonably unique position to comment on ME/CFS and FMT.
I wrote a summary of my FMT experiences here (in the comments section) and it has more detail:
https://www.healthrising.org/blog/2021/08/11/gulf-war-illness-herbs-gut-chronic-fatigue-fibromyalgia/
The more of these study results I see, the more I feel my experiences are representative of these studies. My message would be that you cannot underestimate how powerful FMT treatment can be for ME/CFS symptom management. In my experience, it works within 24 hours. But it has to be done safely.
I live in Australia, where FMT is constrained but not banned. I’ve seen multiple different professors of gastroenterology about FMT and ME/CFS. This includes Prof Tom Borody’s clinic in Sydney. Finding his case study paper was the first time I learned of the ME/CFS and FMT link and dramatic symptom improvement. So I haven’t gone the quack option on this at all.
I’ve also done the full spectrum of FMT approaches. This includes “backyard” enemas (working with my general practitioner in regards to the safety aspects like donor screening) through to the hospital colonoscopy FMT procedures. A recent conversation with my current, outstanding gastroenterologist (a Harvard trained researcher and clinician) backs up my experience. That is, enema FMT seems to be as effective as colonoscopy. A lot of clinics use a combination of both protocols.
I believe donor quality is paramount – I elaborate on that in the above linked blog comments. More specifically, I believe a single athletic donor is more preferable to the diversity of microbiome delivered through multiple-donor-FMT products typically found in a clinic. See the above section on “The Activity Question” for why that might be the case – i.e. exercise stimulation of butyrate-producing bacteria. I don’t think microbiome diversity is the core driver of symptom improvement when using FMT for ME/CFS. By contrast, microbiome diversity probably is key for treating many other GI diseases, e.g. CDI (the “original” clinical use of the FMT procedure).
One significant difficulty is that FMT is still effectively a research protocol for ME/CFS. It may not even be accessible in some countries, at all. Additionally, there is significant expense going down the FMT clinic route. The “backyard” enema approach is on time and on budget. I mention this because this is a major consideration for many long term ME/CFS sufferers. However, FMT carries unacceptable risk if not co-ordinated with a health care provider (e.g. for donor screening, avoiding bowel perforation). I might be being completely irresponsible by saying this, but no one can stop you doing it. The core product is readily available and likely to remain so. But in the day and age of Covid-19 (amongst many other reasons), you’d be mad to go it alone without proper medical service provider support.
For me, my FMT experience all started with a microbiome scan. I’ll send Cort my original American Gut scan results – maybe that can be made available on HealthRising somewhere. My microbiome was a mess. So I reasoned I was a contender for FMT. Perhaps now with this more detailed information (i.e. the above study results), more specific conclusions about microbiome diversity and constituents relative to ME/CFS symptoms could be deduced from microbiome scans.
The fact that symptom reduction does wane over time post FMT (for me at least) always made me believe there was something “upstream” causing problems for the microbiome in ME/CFS. The CoA pathway influence on butyrate-producing bacteria makes sense to me. I wonder how many ME/CFS patients have had (like me) an appendectomy? The appendix is (amongst other things) meant to act as a reservoir for bacteria when recolonising after different GI events.
Finally, recently my gastroenterologist recommended butyrate supplements. As I understand it, the problem is getting the supplement to the right place in the gut without it being destroyed along the way. I’m going to appear obsessed by saying this, but I wonder if enema delivery of butyrate would help for ME/CFS? It would be about a lot more pleasant than FMT, relatively speaking, though perhaps not as long lasting. Butyrate enemas seem to have a history of research in ulcerative colitis treatment, e.g.:
https://pubmed.ncbi.nlm.nih.gov/1612357/
@lono, i could not use the replay button.
thank you so much for the effort it took to write this all!!!
I too had an appendectomy and loosely theorised a few years ago (when I read and a new theory at the time on the usefulness of the previously considered ‘useless’ organ) that having it removed was the source of my problems with M.E. Added to this was the fact that the only thing other than diet that helps me is regularly taking powerful probiotics (if i miss a few days I start to go down hill quickly) – essentially supplementing what the appendix had reserves of. In retrospect it is quite clear than since it was removed at the age of 16 i would catch colds very easily; in my 20’s it was at it’s worst, getting colds and mild flu’s every other other month whilst at college. When I was 30 i had a stressful job that tipped me over the cliff and the rest is my history with M.E.
I looked into FMT treatment here in the U.K a few years back, but the most reputable clinic seemed to have somebad reviews, or were sourcing their the donors unscrupulously, or something, i forget the exact reason. The other option involved being put in touch with donors via a kind of community website setup up by a Redditor, but that seemed too risky, and today post-Covid even more so.
Quick Q. I had this microbiome test done – https://atlasbiomed.com/uk – apparently the results didnt flag anything very bad. Is this test reliable?
Kaz, can you possibly share which products you are taking nd how much? I would love to try. Thank you so much,
Hi Kate, i’ve tried lots of different probiotics (prebiotics make me worse), and it was through trial and error that I found the right formula/approach – some like S Boullaardi had a horrible effect that took a while to correct. I tried a range of narrow spectrum/low-count probiotics to see if I could target a specific issues, but none of those worked. The only ones that seemed to help me were high spectrum large bug count ones, and those took about a month to kick in and do something. I currently take Bio Cultures Complex 120 Billion CFU by Aavalabs and before that i took Renew Life Colon Care (until they changed the formula). I would never endorse them being used because ive read that some have bad experiences with them. I was first introduced to them by a professional who prescribed a low bug count one (Bio-Kult Advanced Multi-Strain). Perhaps dipping your toe in with the low strenght ones would be a way to start? It is so very personal and it took me a few years to work out what was working – there’s no quick route through it unfortunately.
Besides these products I would first just focus on diet. For me the biggest breakthrough came with eliminating all carbs and sugar from my diet. I am esentially paleo, but with some dairy thrown in, and I do not eat any fruit (other than lemons/limes). Only green vegetables, chicken/fish, nuts and goats cheese/cultured yoghurt – red meat I find to be inflammatory, and even fish is questionable these days – and not caffeine at all – even decafe tea/coffee has trace ammounts of caffeine in that really mess with me. I only drink Roiboos tea now. Whaever you do, do it slowly and monitor your reponses/act accordingly, it really is (unfotunately) trial and error and no health care professioanl ive consulted with has been able to do more than ive done through slow and careful testing
What’s “FMT”? I don’t see this term in either Cort’s article, or your own.
Does anyone know of where in the US you can go to give FMT a try? Thank you lono for all the detailed info
Kaz,
That question is a bit above my pay grade. A molecular pathologist might be able to answer it. The company you mentioned does 16S rRNA analysis. There is also metagenomics. Here is an article comparing the two techniques (in chickens):
https://www.nature.com/articles/s41598-021-82726-y#Sec7
Here is a post about metagenomics (less technical):
https://insight.microba.com/blog/how-does-metagenomics-work-solving-the-puzzle-of-your-gut-microbiome/
Christine,
Faecal (or Fecal) Matter (or Microbiome) Transplant.
And about as weird as it sounds, for those new to the concept. But when you’ve been sick enough for long enough, results dwarf weirdness.
Lono- Thank you for the great Post. So interesting. You cannot eat what you want to and get better. This is my bottom line.
My son and I had FMT at a clinic in England with amazing results. Ten people from our local ME group have been, 5 had great results, 3 some improvements and 1 had no improvement. So far we haven’t needed top-ups although others have. I don’t believe it’s a cure but certainly the only treatment that has worked in 32 years.
Interesting study.
I guess the big question is whether these things are contributing to the symptoms of the illness, or rather are a secondary factor.
I think even if low butyrate is a secondary factor it probably is contributing to the symptoms. It does, after all, promote inflammation and has been found in other chronic diseases. So many things are off in ME/CFS that its hard to know what the source is but improving any of them would help I would think
Cort, what if this is like the dauer vs dying enigma.
while it may be normal to have more butyrate, what if there is a reason to not increase it?
sort of like hibernation state keeps person alive
I believe it’s a chicken/egg situation; whichever was first.
It’s a circular concatenation of cause/effect and the important thing to do is to disrupt the vicious circle and apply the remedies when and where applicable.
Maybe we did contract M.E. post-infection…was the infection a consequence of low immune function thanks to microbiome deficiency? 🤔
I wonder why that study used almonds and pistachios? I eat alot of walnuts and pecans.
Almonds (more than 10 ) ,cashews and pistachios ARE the highest in FODMAP while pecans and walnuts are low.
Pistachios & almonds are no-nos on FodMap
Your forgetting about glycophosphate too. Take enough of the non organic nut and if your in America your going to be poisoning yourself at some point.
Can one not just take butyrate supplements?
https://bodybio.com/products/butyrate?currency=USD&variant=34379613044871&gclid=EAIaIQobChMIjMKAkuyO9AIV9MuGCh3tMgUEEAQYBCABEgLDNvD_BwE
Josh and others:
My liver fried out twice this past year but I was on Body Bio butyrate.
How did I feel then- I don’t know..Too many other variables.
I DO test low for butyrate and have low diversity.
The experts who reported this study said they didn’t know if taking butyrate could be negative. I am certainly no expert but cannot see why it would be negative.
I just reordered fresh BodyBio.
People complain the capsules are smelly…
I don’t anticipate swallowing these caps. to be a gourmet experience.
The sulphur-like odor is (to me) no big deal at all.
It does not regurgitate on me.
I’ll try to remember to post again later, in case it is helpful.
Mast cell activation is very bad right now..
That which was tolerated now is not.
So hopefully butyrate will be ok.
I
Very interesting. Thanks Cort.
In one of my previous Gut/stool tests the results for Aerobic bacteria were:
“E-coli 0.09%,” and the reference range was “70-90%”.
Most of the aerobic bacteria should have fallen into one of 4 groups, but it didn’t.
There was 99.83% “Other” bacteria noted.
Also, the “Aerobe/anaerobe index was only 0.3, where as the reference range was 1.0-2.0
I had severe malabsorption at that time, that was improved with digestive enzymes.
wayne, may i ask wich gut/stool test you had? and is on the result of it written wich digestive enzymes to take and wich malabsoptions you had? I live in belgium, 98-99% bedridden so if i can, i send my stool to there. but i need to know wich malabsorptions and digestive enzymes to take to show to my gp.
And may i ask, did you feel better afterwards? a bit, much, a lot? ofcource i do not know on wich level you function. And if better, what symptoms got better?
thanks a lot!!!
I took butyrate supplement and shortly after began feeling extremely sick…sore throat, fatigue worsen, pains, developed congestion, drainage and severe coughing. Super intense and hard to deal with, and matches the same symptoms I had in 2001 when I had my “triggering event” that put me into the world of CFS. Had a similar experience with LDN. Could not tolerate, so had to stop. I believe my guy is severely out of balance but in order to fix it wld need such an intense immune response that I almost need to be hospitalized in order to go through it.
Such an interesting article Cort. As a long term ME/CFS patient I have recently undertaken gut permeability tests, expecting to see “leaky gut syndrome”. To my doctor’s surprise the test revealed “poor nutrient uptake from the small intestine”. This article certainly gives me a deal of hope for future research in the role of the gut in ME/CFS.
Ha! Poor nutrient uptake from the small intestine….Fascinating. What was treatment recommendation?
Poor nutrient uptake is currently on my radar; I read recently that candida overgrowth can cause a film to the stomach lining which reduces absorption of nutrients?!
may i ask wich tests? thank you!!!
I am on a Amino Acid compounding prescription because I cannot digest or synthesize protein. It contains butyrate. The reason I know this, is because the compounding pharmacy issued me the mix one time that was missing butyrate because of a shortage issue and they advised me to buy it in supplement form & take it with my Amino Acids. I found the Genestra brand Butyrate complex from an online health food site. I can’t remember what site, but a person could do a internet search to find it in your own Country.
Why does all the ME/CFS research continue to ignore that parasites exist, and they can and will cause the same symptoms?
I don’t know why parasites are not studied as I would think the same: they’re pathogens, can interfere with nutritional absorption, cause gut problems. Giardia is classified as a parasite and studies show it can cause ME/CFS. This is a small field – and it shows.
I always wondered if the Giardia aided in the move to CFS or whether it was the antibiotics used to treat Giardia (Flagyl or metronidazole)
The Parasites are a big issue. Most Physicians do a stool test and say no. When you know and see them. I do not get it. The Probiotics I take do wonderful with this issue. As tests do not always show it up. That may sound crazy but I have had at least 10-12 tests. They all say no on Parasites. This is from diff Labs???/
I was diagnosed with ME/CFS in early 2016 after getting dengue fever, a kidney infection, taking antibiotics that then gave me gut problems followed by some other virus, all within a four-month period. I was moderate to severe over a 4 year period, trying everything I could to finally see some progress and then another year to get to 95% recovered. Around the 4 1/2 year mark, I found a naturopath that specialised in gut issues. He found SIBO and it took just under a year to resolve PEM, POTS, restless legs, and chronic pain and somewhat improve my brain fog. I recently did an elemental heal diet (gut healing) and my brain fog seems resolved now. To help with insomnia I used CBD oil. It wasn’t a quick fix and I know people recover in all different ways but I still highly recommend anyone who has ME/ CFS to try to fix your gut as a way to heal.
According to my microbiome testing, I have good butyrate production.
What did they find out about oxalates? Were oxalate degrading bacteria killed off by patients’ use of antibiotics, which can cause a cascade of health problems?
They did not find increased antibiotic use in the ME/CFS patients – so discarded that as a source of their microbiome issues. I didn’t see anything about oxalates. Acetate, however, was reduced.
there is a researcher studying college students and trying to see which eventually get cfs me
would not there be info to show looking back if gut issues were pre or post?
Hi,
The gut issue is at the heart of Ken Lassesen blog https://cfsremission.com/
He has succeeded in restoring his health through gut alteration.
In other notes, I improved my health significantly with and FMT 1.5 years ago, however in the last 3 weeks, I have been wiped out again to a level I had not felt since before the FMT.
I worked with Dr Cheney and he recommended the BodyBio liquid butyrate (but it gets expensive)
Dr Cheney
thak you verry much, am desperate
Hi Konijin,
I feel your desperation. I am in the midst of my worse relapse in 8 years, but I am pretty sure I can get much better again, although there are days currently where I wish I were dead. As I said above the FMT helped me like nothing else, and this recent relapse is leading me back to trying to figure out why it wasn’t a better cure. So, I am now focusing on the Briggs protocol and SIBO and the information in cfsremission.com along with the goal of doing another round of FMT. I recommend starting to read the website https://thepowerofpoop.com/ to familiarize with the idea of FMT and the Briggs protocol. The basic steps I am looking to do are Heal the Gut, which includes removing biofilms, killing off bad bacteria, and trying to restore good bacteria (Briggs Protocol and cfsremission are good places to start) as well as diet. Patience and stress reduction are important . This is my my biggest challenge as I am going through divorce, had to move to a new city to be close to my kids, and have no friends and I am trying to keep my work from home job which has become much harder with this relapse. The next hardest part is finding and screening a poop donor, but thepowerofpoop has some more suggestions. It has to be a relationship as you are going to want to have access to this persons poop for 2 weeks to 6 months. That does not mean you do a transfusion every day for 6 months.
If others are interested in working towards this goal, I would like to find like minded people to work with and set up a group to work together as this process takes energy and a functioning brain both of which I have limited amounts of at the moment.
Was just going to mention Ken’s blog.
It was through his blog I learned of Miyarisan, a Clostridium bacteria that both prevents C. difficile AND produces butyrate. Manufactured in Japan. A bit hard to get. We take it.
The science is becoming clearer that a gut dybiosis is the root cause of many neurological diseases and the understanding that the same gut dybiosis is responsible for other chronic health conditions..
There are protocols to address the gut dysbiosis. First, clear the garden of weeds and poisonous plants. This is done with an elimination diet and natural antibiotics. With a clean soil we can plant good guys. Fermented foods and lots of prebiotics. Diversity is key. Phil Spector of Kings college has shown how quickly you can change the microbiome.
The key to butyrate is fibre and resistant starch. Fibre is the key. Fibre gets broken down into short chain fatty acids. Butyrate is a SCFA..
Resistant starch is baobab powder, green bananas, reheated rice and potatoes. The starch can’t be digested in the stomach but passes into the colon where its digested and butyrate is generated..
Ive healed a chronic autoimmune disease with these protocols and it continues to help my severe long covid..
Thanks David for sharing that.
I agree with you Cort that bacteria in the gut play a role in ME. I even thought for a while that this was the cause of ME. Bacteria produce endotoxins, this could explain everything.
I also do not rule out enteroviruses as a cause.
I’ve been wondering if sodium butyrate supplements would be of any help. But I was thinking making enemas or suppositories out of them to try to get them where they should be. I wonder if anyone has tried this yet?
i take Pure Encapsulations SunButyrate-TG Liquid it is a good brand & 1 tsp a day works. I definitely normalized my levels with that. (USA)
look for guidance from GAPS Diet
Gut and Psychology Syndrome by Dr. Natasha Campbell-McBride, MD, MMedSci (neurology), MMedSci(nutrition)
A careful low and slow approach to fixing the gut. Cort just published information about the strength of using fermented foods for gut improvement and this program follows that theory but with VERY careful increments to get there.
I just ordered butyrate today. Never heard of it before. I have intermittent IBS issues, and had it pretty severely about 5 years ago. Yesterday I read something that dandelion leaves are a good source of butyrate. Interestingly enough I’ve been eating dandelion leaves from the grocery store a lot in the past few months. It’s like I can’t get enough of them sometimes! Anyway, this is really interesting. Thank you again, Cort. Fingers crossed yet again that this may help us get even closer to answers for MECFS. Hopefully no adverse reactions to the supplement, nothing stood out for me when I did a brief side effects search. (However, 1 supplement company put a lead warning on their label for butyrate! ??? )
what is the link with post viral fatigue and butyrate? I see no connection.
Can someone explain this?
Many patients I know had had problems with their bowels before they got ME.
I’ve supplemented with butyrate and it worsens my digestive distress.
Hi Cort, amazing article, thank you! I would love for you to look at the link between low acetate and not only butyrate but the urea cycle as well as oxalate issues (because the body splits oxaloacetates into acetate and oxalate to get the needed acetate).
Studies have found out that acetate is produced by most of the enteric bacteria such as Lactobacillus spp., Bifidobacterium spp., Akkermansia muciniphila, Bacteroides spp., Prevotella spp., Ruminococcus spp., and Streptococcus spp and also derived through dietary input. Notably, it is a metabolite for combining with CoA to create the highly essential acetyl-CoA, which is a critical cofactor for the synthesis of
every single acetylated metabolite.
eg.
Acetylcholine (involved in cognition and muscle activation).
Acetyl-L-Carnitine (involved in burning fat for energy, or “beta-oxidation pathways”).
Acetylation of histones, performed by Histone Acetyltransferase (HAT) (the opposite
process of “histone deacetylase” (HDAC) – both are intricately involved in DNA
transcription.
N-Acetylserotonin (involved in neurological pathways).
.. and a vast number of others.
I´m quoting Joshua Leisk´s paper “A New Hope” on CFS/ME, which you´re probably familiar with. I think the low acetate is a key player in this mess.
Thank you for all your hard work man!
link to above paper
https://www.researchgate.net/publication/350956432_CFSME_A_New_Hope
I think that’s interesting because it kind of makes sense of the findings from Stanford about fermented food supplmentation not increasing diversity, but increasing the hospitality of the gut TO diversity. It might be that the lactos preduce the acetate that then foster other important critters.
Byterate is the key. Taking byterate supplement is harsh on the digestive system, because it’s an acid. So, I am 55 and have complete red, mutations from father and mother, of the FUTs gene. This gene is responsible for feeding the byterate making bacteria. I battled with chronic fatigue, especially after eating, due to very bad compromise to my gut lining, leaky gut. Itchiness, fatigue, cold extremities (feet), inability to handle thiols (sulfur) from food or supplements. Extreme chemical sensitivity. My blood brain barrier was weak. Immediate flood into my brain, if I absorbed something into my blood stream, such as chemicals from skin lotion (absorb into blood stream; why do you think we can take magnesium supplements via dermal application?) I’d have canker sores all the time that would take 10 days to heal, and they would come so easily, too acidic of food to a slight nick in the mouth. I was a mess for majority of my life. I spent thousands on hundreds of supplements to try to solve including very expensive probiotics, all sorts of brands, prescription and not.
I was suicidal from the extreme health crisis I evolved into.
So, I now take one thing. I eliminated everything else I was trying or tried. I turned around 180 degrees and I am no longer dealing with the issues I mentioned above.
All my research led me to byterate. I experimented with ways if increasing my byterate, from potato starch, to twice baked and cooked potatoes, to probiotics for byterate, to Jerusalem artichokes, to a myriad of prebiotics. The one mack daddy that beat them all out was high quality, high fiber, green banana powder. I tried different brands and found one brand to be better than the rest. I take six tablespoons a day in a smoothie. It resolved all issues. That is a lot of banana powder. But it is all I take. Thousands in medical tests, supplements, mental gymnastics to figure out the balance daily. None of that anymore. It’s miraculous for me. I won’t mention the brand, because this is not about marketing. But if you want to figure it out, pay attention to the fiber content of the green banana powder. The one I take has much higher fiber content. I tried various brands and this brand really madeva difference. Others were much weaker in creating the response I needed in my body.
It has to be the correct prebiotic and enough of it to build up your byterate. I have experience with identifying low byterate as my main issue but attacking how to truly build it up in my system. If I miss three days of my regimen, my body starts degrading. Canker sores heal litrlerally in one day. My dentist number my mouth and he had me bite down. He didn’t see my cheek got caught and a major gash formed. He put in stiches to hold it together. My mouth healed so fast, I myself, took out the stiches the next day. Imagine what that healing capability is doing for the gut lining, for the blood brain barrier. Byterate is instrumental as the ‘thing’ that is used by the body to affect the health of our gut lining. It turns on and off certain genes too. Magic stuff here and I’m proof in the pudding that this research is bang on.
Corrected my misspelled words and resubmitted this post.
Byterate is the key. Taking byterate supplement is harsh on the digestive system, because it’s an acid.
So, I am 55 and have complete red, mutations from father and mother, of the FUTs gene. Meaning I’m completely lacking the typical response from this gene. This gene is responsible for feeding the byterate making bacteria. I was the colic baby that no one could figure out why. My FUTs was the culprit.
I battled with chronic fatigue (body would completely drain of energy and I’d feel like I was just hit by a Mack truck, especially after eating, due to very bad compromise to my gut lining), leaky gut. itchiness, fatigue, cold extremities (feet), inability to handle thiols (sulfur) from food or supplements (cysteine would create crazy reactions in my body), extreme chemical sensitivity. My blood brain barrier was weak. Immediate flood into my brain, if I absorbed something into my blood stream, such as chemicals from skin lotion (absorb into blood stream; similar to taking magnesium supplements via dermal application.) I’d have canker sores all the time, since teenage years, that would take 10 days to heal, and they would come so easily, too acidic of food to a slight nick in the mouth. I was a mess for majority of my life. I spent thousands on hundreds of supplements to try to solve including very expensive probiotics, all sorts of brands, prescription and not.
I was suicidal at the worst point from the extreme health crisis I evolved into.
So, I now take one thing. I eliminated everything else I was trying or tried. I turned around 180 degrees and I am no longer dealing with the issues I mentioned above. It’s been two years now.
All my research, from 30 years, led me to byterate. My challenge, how to increase it in my body? I experimented with ways if increasing my byterate, from potato starch, to twice baked and cooked potatoes, to probiotics for byterate, to Jerusalem artichokes, to a myriad of prebiotics, to high dosage from pills. The one mack daddy that beat them all out was high quality, high fiber, green banana powder. It is a resistant starch. With it, I tried different brands, spent money, time and effort and found one brand to be better than the rest. I take six tablespoons a day in a smoothie. It resolved all issues. That is a lot of banana powder. But it is all I take. Thousands in medical tests, supplements, mental gymnastics to figure out the balance daily. None of that anymore. It’s miraculous for me. I won’t mention the brand, because some will think it suspicious to recommend a particular vendor. But if you want to figure it out, pay attention to the fiber content of the green banana powder. The one I take has much higher fiber content. I tried various brands and this brand really made a difference, that I physically noticed. Experience is the Mack daddy of finding out the truth, for our bodies. Others were much weaker in creating the response I needed in my body. There ability as a resistant starch was not as powerful, for whatever reason.
It has to be the correct prebiotic and enough of it to build up your byterate. It took dna and 30 years of struggles to identifying low byterate as my main issue but attacking how to truly build it up in my system was the next step.
If I miss three days of my regimen, my body starts degrading slowly.
Now, canker sores heal literally in one day. To be honest mouth cuts do not develop into canker sores anymore. They heal in a day. My dentist numbed my mouth this year, first visit after a year break from letting COVID settle a little, and he had me bite down, to test my bite. He didn’t see my cheek got caught and a major gash formed. My mouth was numb, I didn’t know. He put in stiches to hold it together. My mouth healed so fast, I myself, took out the stiches the next day. He was to take out the stiches a week later. Imagine what that healing capability is doing for the gut lining, for the blood brain barrier. Byterate is instrumental as the ‘thing’ that is used by the body to affect the health of our gut lining, our mucous membranes. It turns on and off certain genes too. Magic stuff here and I’m proof in the pudding that this research is bang on.
A more easily sources resistant starch that may be close to banana powder (or for those who prefer to diversiy) is potato starch (uncooked, mixed in smoothies).
Hi Mark,
I looked at most brands of green banana flour. Most of them have about 10% fiber. The only one that I found with much higher content was Jonny’s Good Nature. Is this the brand that you use?
How can they get 75% fiber in their product when every other brand has 10%. Is their label truly accurate?
I’ve got banana trees in the back yard. Green bananas are pretty rough to eat (like chalk). I’m not sure if they are as good as the powder (in terms of the amount “active ingredient”).
I’ve got no idea of the ease or cost of sourcing these (depending on where you live). You can peel and freeze them. I’d be 99% sure they don’t then have any problems with the freezing as far the fibre goes. From frozen bananas, some sort of smoothie in a blender is probably the go. That’ll be about a thousand times easier than eating them raw anyway.
Marc, thank you for all your information and for sharing your story. Did you have extreme constipation with all your symptoms? Like all your gut motility was gone? Or the other way around? I’ve tried alot of resistant starch and hasn’t worked so far, only clogged it up further. But I agree, simple is better as I was doing lots of other things with that. There can be one thing/product/amount that turns it all around, after spending tons of $$. It’s so individual and timing is important.
Thank you for sharing this info Marc. How incredible that you managed to get to the root cause of your lifelong health problems with banana powder. Am definitely trying some!
Your insightful comment about FUT polymorphisms really struck a chord with me and reminded me about Travis Craddock’s research where he looked at 23&me records for nearly 300 patients. Nearly 80% of them had genetic polymorphisms in the genes for mucus production. I didn’t realise just how important MUC2 and FUT are for maintaining a healthy gut barrier and microbiome. Gut problems have always been such a major part of my illness, plus asthma and low resilience to all sorts of viral and bacterial infections since childhood, and so genetic issues are likely.
As so many of us also have MTHFR mutations, if we have leaky guts we probably don’t have the greatest ability to clean up the mess.
Careful diet seems to be essential for me. After 25 years of experimentation, my best diet seems to be veges, fruit, wholegrains, nuts, legumes, a little meat, no gluten and not much dairy or refined sugar. I guess that is something akin to a Mediterranean diet.
Thank you for sharing. My issues with sulfur have direct correlation with my genes and my sulfur channels. Mutations. Healthy byterate levels has for some reason had a positive effect on better handling sulfur. I can eat broccoli and garlic with no effect now. I’m still avoiding whey, the runoff in sour cream. I’ll eat sour cream but pour off the whey. If I eat protein bars heavy with whey I can handle one or two but multiple everyday and still my body starts reacting again. Without the healthy byterate levels and I’m a wreck for a few days as my body eliminates, processes, the sulfur. Today, I eat as much broccoli as I want, all the garlic I want and I’m reaction free.
Gene mutation is a real thing, that affects a lot of people. A well oiled sulfur channel helps get healthy levels of glutathione working in the body to help detox the body from heavy metals and chemicals we take in through food, water, air we breathe.
BUTYRATE. Not byterate. I thought when you posted your 2nd post to correct your spelling you’d correct that, but anyway. 🙂
Molybdenum will help your sulfur issues. In fact, it’s THE trace mineral that helps convert sufites to sulfates and also handles sulfur in all sulfur foods, including the methionine and cysteine in meats.
I have done 15 min.-45 min. of aerobic activity on my recumbent machine nearly every single day since 1994 when I was diagnosed with FM. The slow recovery of said activity was one of the ways I discovered I had FM. (It took 6 wks to recover from the damage to my muscles!) But I gradually built-up the length of my exercise program minute by minute. Over the years I never saw any connection between my gut/IBS symptoms and exercise. It seems more to do with inflammation and food..perhaps a response to mast cell activation or malabsorption. All I know is Gastroenterologists need to increase their curiosity and help their patients fight these life-hindering symptoms!
Inflammation is caused by compromised gut lining, vicious circle. Causes it and is the result of it. Byterate reduces inflammation. Try grounding too. Greatly reduces inflammation. It’s what it is known for. Touch mother earth to ground the body.
Thank you for this information Mark. I also have a very compromised gut lining and tested low for butyric acid. Did you have to build up to those 6 TBS a day of green banana powder? I’m reading online that you need to take it slow in the beginning and gradually increase it as you can experience some gas or bloating as your gut flora evolves. And what about raw green bananas or plantains themselves? Thanks.
Hi Marc, would you share which brand? Much appreciated!
Maureen- I was an athlete and ran 10 miles a day when I became sick in 1987. I did quit running but kept up when I could walking long distance. Also taught Yoga before. I have found after this time. I have to do most of my exercises before I get out of bed. Yoga and then walking 5 days a week. I am sure it has kept me healthier. I do what I can when I can. Even if it means breaking the times up. I know it has helped me. Thank you for your comments.I know the exercises help me think better and calmer.
Marc K.
I have found one source of high resistant starch green banana flour (10g) and probably it’s the one you found. Then I found one more at 13g from Haiti. It’s ZestofMoringa,com, but it’s really expensive to get this to be shipped to Canada. I would love to support the people of Haiti, so I bought both. We’ll see which works better.
Ha! I just realized that you’ll have to search for “green banana flour” on the ZestofMoringa.com website to get past the penis enlargement products! Here’s the direct link:
https://zestofmoringa.com/search?type=product&q=green%20banana%20flour*
I have ordered it and we’ll see what happens. I hope it helps my fibromyalgia, since I don’t have a penis.
Ann~ I wish we had a laugh icon so I could reply to your comment. I too will be checking out green banana flour, but I’ll be alert to any ads that may not apply to me since I am also female. Thanks for the chuckle. 🙂
Thanks, Tina, I have just discovered this blog and I thought I might be able to add something, only to see that I had linked to something that might offend some readers, so I had to make a joke out of it.
I am a retired nursing professor in British Columbia and I know now that I have probably had fibromyalgia for twenty to thirty years. A slow and insidious ramp up for me. I also now know that my mother had it and now my daughter has it.
I have so much to read here. Being an overeducated nurse, my first thoughts were modern western medicine. After being, ahem, totally screwed over by this, I realized I’m on my own. I won’t go into the bazillion of things I have tried because it would fry your brain just to read the names of all of them.
Anyway, now I’m on LDN 6 mg (helps a lot, but start at .25 and don’t let anyone tell you different), PEA, quercetin with bromelain (natures’s benadryl, so take at night), melatonin, lots of vitamin D, microdosed psilocybin, and red light therapy. The definitive book on this has been updated and is on the web here:
https://theenergyblueprint.com/red-light-therapy-ultimate-guide/
At the moment, I am intruigued by this study:
https://pubmed.ncbi.nlm.nih.gov/30076785/
which, if it turns out to be true, would blow the lid off. It says that ubiquitin is essential for the functioning of all eukaryotic cells. Bacteroides fragilis is a normal member of the intestinal flora. They have discovered that some people have a B. fragilis variant that has undergone horizontal gene transfer and now produces a ubiquitin mimic (66% similar) to our own ubiquitin. This may cause our own immune system to attack our own ubiquitin. They conclude: “Molecular mimicry of human ubiquitin by BfUbb could be a trigger for autoimmune disease.”
Wow. But how could you remove this variant? And how could you erase the immune system’s memory of it? These seem to be the big questions.
I have taught research methods to undergrads and graduate students and this looks like a well done study. I haven’t seen any follow up, but I sure wish someone would do so. I cannot for the life of me get anyone around here to even take a look at it, so I’m putting it out here.
Oh, yes, one more thing. Bananas. If you’re really serious about this source of resistant starch, look here:
https://www.alibaba.com/product-detail/Green-Banana-Flour-Australia-Green-Banana_1600083959224.html?spm=a2700.7724857.normal_offer.d_title.5cb297a4iX2dxG&s=p
I’m going to try a small amount of green banana flour first, but if it works, I’ll be ordering a couple dozen kilograms of this stuff.
Hmmmm, this might explain why the Wahls Diet (eating 9 cups of fruit and veggies daily) isn’t working for me. Have been at it for six months and boy… my bowels! Yikes. Despite having a feeling that perhaps there was a sliver of hope for about a day a week, but the other six days… pffffff.
So now I’m wondering if a low fiber diet might work better for us? It turns out that the thing I eat are mostly high in fiber such as cauliflower, leeks, carrots…
Any suggestions? Thanks y’all – hang in there.
My usual skeptical self thinks the reduced butyrate could well be another symptom of ME/CFS rather than the cause since it doesn’t easily explain the sudden onset of ME/CFS or PEM. It could be a perpetuating factor (trap), but such line of thinking also requires some complicated logic. This well done study could offer another clue to the mechanisms of ME/CFS though.
When one looks closely at any case of ME/CFS, 95 times out of 100, it’s usually not ‘sudden onset’. There were several smaller issues that stressed the immune system, then the patient got hit w/the ‘issue’ that was the straw that broke the camel’s back.
And stress itself depletes the good bacteria, alters gut function.
Dr. Will Bulsiewicz, a plant based GI doctor, talks a LOT about butyrate in his book Fiber Fueled. He discusses the GI tract and how to get and keep it healthy in great detail. It’s worth a read. Dr. B’s website is here: https://theplantfedgut.com/ And here is a recent Q and A here: https://www.youtube.com/watch?v=ZwvbPYz82AE (the subject is constipation, not butyrate, but it will give you some idea of who he is.) Here is another interview where he discusses butyrate a bit. https://darinolien.com/82-dr-will-bulsiewicz-on-how-to-heal-your-gut-with-plant-based-fiber/
Great post and comments.
Is the best source of resistant starch Green bananas!?
They do seem to be a wonder food.
I have just started eating them whole. Tastes like cardboard! Two a day.
Tina fiber fueled is one of my favourite books!
Hi Wallace,
If you like, you can make your own green banana flour:
https://www.thehealthyhomeeconomist.com/homemade-banana-flour-recipe/
but you wouldn’t know how much resistant starch was in it. I may resort to this, but at the moment I’m going to try some store-bought to see if it works for me.
Thanks Ann.
I dont see the point of buying it as a powder when Green Bananas are freely available.
Its supposed to help with restful sleep due to its high B6 content.
Does it help your sleep?
Hi Wallace
It hasn’t arrived here yet. Postal Service to Canada is like asking for it to be sent to the North Pole. When it arrives and I try it, I will post my experiences right here, so stay tuned.
Hi Wallace
Canadian Postal Service now says I will get it on December 7. We’ll see. There is more flooding forecast for British Columbia. It’s a mess out there.
Hi Wallace:
The green banana flour arrived and I’ve had 2 days to check it out. Be sure to begin slowly or you’ll have pain! I didn’t like it because it has an odd taste, but worse, that taste keeps coming back up.
I think I’ll stick to green bananas at the store. They don’t taste like much but at least they don’t bounce back!
Cort,
I can definitely ascribe occasional (even vast) amounts of antibiotics prescribed pre- and post-operational as a major factor in causing my gut dysbiosis.
Additional to and post-dating infections of Giardia, Malaria, Bilharzia and at least 3 different dysenteries, both amoebic and bacterial.
But then, I can also trace my symptoms of ME/CFS right back to a very difficult birth closely followed by Measles Meningitis and later on, Acute Otitis Media.
Not about to relate my autobiography here, but now in the run up to my 75th birthday, I am beginning at last to heal my gut with the help of all this new knowledge.
I said once to a trusted GP, a rare gem now prematurely deceased 🥲, I should be in perfect health by the time I die.🤞
Where there is Life, there is Hope.
Never despair….
Look after your microbiome and those critturs will look after you.
Let food be your medicine and medicine be your food.
Sounds to me that the low Butyrate is likely a result of the chronic “Flight/Fight/Freeze” stress response which takes the “rest & digest” (digestion and assimilation of nutrition) offline. It’s back to the higher level dysfunction between the brain and nervous system and how the nervous system affects all these various components in our bodies. Digestion being just one of them.
Hi Marc, would you share which brand? Appreciated!
Thank you Johnson for a high value article, and hi and thank you to all of you commenters.
Regarding butyrate and IBS; At it worst my IBS was bleeding. I tried once to massage the area in my descending colon where it started bleeding. Today all symptoms are gone. This is mainly caused by ingesting guar gum, which feeds butyrate producing bacteria, but also regularly eating cooked cauliflower, celery root, and pointed cabbage. For some reason I have found pointed cabbage the better when it comes to cabbage types. Sunfiber and guar gum is the same, but the latter is cheaper.
Regarding fecal therapy and probiotics I believe fecal therapy is better. I would like to emphasize four things. To get the microbiome to stay in your colon you need to get it all the way around to the ascending colon and down to the Cecum. The reason is that the Cecum is a reservoir for the colon bacteria, just below where the small intestine is attached, constantly supplying the whole colon. Just inserting fecal matter to the descending colon will not supply the whole colon. Thus it will likely be lost over time. So probably a 3 or 4 day fast to empty your colon before doing fecal therapy would be useful to get the microbiome all the way round to the Cecum. Yes, it would mean that you have to start with standing on your head, then laying on the right side before ending with standing up :). Third, the microbiome is adapted to our individual genetics. Coming from our mothers in the first place. So the best provider would probably be from a healthy mom living a healthy life. Your children could be your next best, but microbiome genetic adaptation to your own body will take some time. Last, you have to feed your new bacteria with fiber rich foods, like the ones I have mentioned, and keep on doing it for the rest of your lives. For chronic fatigue reasons I have tried the regular fecal therapy, but from what I know now I am about to do what I have described above.
Last, regarding leaky gut syndrome. All stress causes leaky gut. Daily chronic stress, high intensity training, and severe physical trauma like large third degree burns. This is why some athletes take colostrum after high intensity workouts. Some years ago I read a scientific paper which told about positive results treating severe leaky gut caused by large third degree burns with colostrum. Also, we are all born with leaky gut which closes within two to tree days, because of colostrum in the mothers milk.
As for chronic stress and all the havoc it creates, I suspect this is where the whole thing starts. More specifically with the right side amygdala. During prolonged stress periods the right side amygdala starts to grow and will have an abnormal functioning where it amongst other things gives you an oversensitive stress response. Also meaning it self creating inflammation, hormonal imbalance, and keeping the sympathetic nervous system constantly on high gear, never giving us the opportunity to be in “rest and digest”, run by the parasympathetic nervous system/vagus nerve. For those interested look up amygdala reprogramming and neuroplasticity. I’ve been doing primordial sound meditation and the second track on the Holosync Super Longevity cd from centerpointe-dot-com for a month now and it works “like the dickens”. I literally experience the brain changing and a clear improved stress response. In studies they have done MR on participants before and after three months of meditation where they clearly see the reduction of amygdala size.
All the best
Thanks for all the great ideas Monti! 🙂