


The RECOVER Review presentation explained how to have a breakthrough in treatments for long COVID (and therefore possibly ME/CFS/FM)
Nothing yet in long COVID, or PASC, has better illuminated for me the difference between the chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) field than the recent RECOVER Research Review talk on biomarkers and long COVID. (Transcript here.)
The participants in the hour-and-a-half talk were impressive. An infectious disease doctor at the University of California San Francisco, Dr. Peluso, was, prior to the emergence of long COVID, focused on “long HIV”. Dr. Abdel-Mohsen of the Wistar Institute is a specialist on how the immune system responds to viral infections. Dr. David Walt is an award-winning nanoscience and nanomedicine inventor. Dr. Grace McComsey at Case Western Reserve University is an internationally known researcher in the field of the metabolic and cardiovascular complications of HIV. All appear to have glommed onto the possibility of long COVID quite early.
With long COVID, we’re in a different world – a bigger, more complex and more precise world. We’ve talked about biomarkers for chronic fatigue syndrome for years, but after Peluso’s talk I wondered if we have, really.
Dr. Peluso on Biomarkers…and an Explosion in Therapeutics
Peluso began one of the first long-term long-COVID studies in April – yes, April 2020. The man clearly knew what was coming. Since then, he’s enrolled hundreds of long-COVID patients into his LIINC study – which is now part of the RECOVER Initiative.

Symptoms are too variable to provide good endpoints. Biomarkers are urgently needed.
Symptom Assessments Don’t Cut it
The first takeaway from his talk was that using symptoms to characterize patients just doesn’t work. Too many variations in symptom presentation – even in this single virus-triggered illness – are present to use them. There’s lots of variability over time within individuals, and even more variability between individuals. That makes it hard to use “patient-reported outcomes” – those symptom assessments we see so often in ME/CFS and FM – as core outcome measures for long COVID.
Of course, this problem has been recognized in ME/CFS for years and is regularly trotted out as the reason for low pharmaceutical company interest. This is a big deal – so big that Peluso said, “we really desperately and urgently need other measurements that we can make to supplement the clinical data that people are telling us”; i.e. they need biomarkers!
Obviously, we’ve hardly scratched the surface of biomarkers in ME/CFS, as four different types of biomarkers exist – none of which I’ve ever seen differentiated in this disease.
- Mechanistic biomarkers – measure what’s gone wrong – as in high levels of the IL-6 cytokine. IL-6 wouldn’t be a diagnostic biomarker because high IL-6 levels are found in other diseases, but it would indicate the extent of the illness.
- Diagnostic biomarkers – a biomarker that’s unique to long COVID; if someone has this, they have long COVID.
- Predictive or prognostic biomarkers – biomarkers that suggest someone is at risk from long COVID, or that someone who has long COVID is at risk of having a severe case of long COVID.
- Surrogate biomarkers – the holy grail of biomarkers – a biomarker which, when you change it, the patient gets better or worse. Surrogate biomarkers pretty much make clinical trials a snap; all you need to do is measure the biomarker to see if the trial is successful.
The HIV Saga Tells the Tale
Peluso said that for most of the ’80s and part of the ’90s, diagnostics and therapeutics for HIV really struggled because of the lack of biomarkers. People were trying everything under the sun – almost always unsuccessfully – for one reason and one reason only – the field lacked accurate biomarkers. Clinical trials, that were based on the same types of inaccurate endpoints that are now available for long COVID and ME/CFS, had to be enormous to tease out even small positive effects over time.
Peluso predicted that finding good biomarkers will lead – as they did in HIV/AIDS – to an explosion in therapeutics.
Everything changed, though, when the biomarker for HIV – a surrogate biomarker (plasma HIV RNA levels) – was finally discovered in the mid-90s. With that, they had a target they could use in clinical trials, and bam – the field just exploded and one treatment after another started popping up.
Peluso compared the situation with long COVID now with the early stages of the HIV epidemic. They have some ideas about mechanistic biomarkers that can demonstrate how severe the illness is but no diagnostic, predictive, or surrogate biomarkers.
Possible mechanistic biomarkers in long COVID:
- A persistent virus – Dr. Maureen Hanson is studying exosomes in ME/CFS and Goetzl and Waltz at UCSF found – apparently to their great surprise – the SARS-CoV-2 coronavirus proteins months after infection in the exosomes of long-COVID patients. People with more severe neurological symptoms had more proteins in their exosomes.
- Inflammation – a 120-person study that used an ultrasensitive assay found that people with long COVID had higher levels of inflammatory markers such as interleukin 6, TNF-alpha and IP-10. The sicker they were, the more markers of inflammation they had. Plus, people with higher levels of inflammation had reduced exercise capacity. As these findings have been replicated in other studies, it’s now becoming clearer that inflammation, as suspected, is playing a role in long COVID.
- Other possible mechanistic biomarkers include autoantibodies, coagulation, cortisol, microbial biomarkers in the gut, and exercise study results.
It took 15 years to find a surrogate marker for HIV, but Peluso believed that all these biomarkers – mechanistic, diagnostic, predictive, and surrogate – will be developed more quickly with long COVID (as well they should given the enormous growth of technology since then.)
He predicted that the studies (remember he’s in the RECOVER Initiative) are going to “really increase in complexity over the next year” and when they do, the biomarkers are going to become clearer.
The big news, though, is that the production of biomarkers – particularly surrogate biomarkers – should unleash an explosion of treatment trials and ultimately, good therapeutics for long COVID and, I suspect anyway, ME/CFS (see Conclusion).
Dr. David Walt – A Persistent Virus (or Not?)
Talk about using cutting-edge technology. Dr. David Walt (an inventor in the US National Inventors Hall of Fame) described the technique he’d developed which allows him to “literally count the number of molecules present”. Say bye-bye to all the background signals that cause chaos in standard Elisa studies, and say hello to being able to detect much more – like 1,000 to 10,000 x more – with this new technique.
He followed 37 people diagnosed with PASC and 26 patients who had come down with COVID. Trying to determine if the virus persisted, they looked for four aspects of the virus (the nucleocapsid portion of the spike protein, the S1 fragment of the spike protein, and the full spike protein) and did ultrasensitive assays of 10 cytokines. None of the patients they followed had been hospitalized.
The persistence of the spike protein in the first long COVID study suggested an active replicating virus was still present. (from NIAID)
To their surprise, they found evidence that the spike protein persisted in 65% of the long-COVID patients – but not in the healthy controls. That indicated that a persistent viral reservoir of actively replicating virus must be present in a large number of the long-COVID patients. It also indicated that a viral reservoir was not causing problems in many people with long COVID.
The cytokine assays – as they have often been in ME/CFS – were totally unrevealing; i.e. no difference was found even in the supersensitive assays between the long COVID patients and the controls.
Beta Testers Requested!
Then came the fly in the ointment. They’d just tested 600 post-Omicron patients from the RECOVER study and found much less evidence of the spike protein; i.e. much less evidence of viral persistence! Was it because of the different variants? Or god forbid, did the reagents they used in the two studies have “a slightly different” specificity? They should know more shortly as they’re continuing to test different batches of patients from different groups – and that’s the good news. In ME/CFS, a problem like this might have ended this effort, but they have the resources to figure this out and they will.
Meanwhile, the presence of the spike protein is being used to assess effectiveness in the Paxlovid trial.
Dr. Abdel-Mohsen – The Gut-Brain Connection in Long COVID (and, of course, ME/CFS/FM)
The next presentation asked whether the virus had altered the flora of the gut to such an extent that leaky gut had occurred, that evidence of systemic inflammation was present, and most importantly, that the metabolism had been affected.
We don’t often think of the gut in terms of metabolism, but the gut – with its production of many metabolites – plays a huge role in our metabolism. If crucial metabolites (such as tryptophan) are not being metabolized properly, big problems can result.
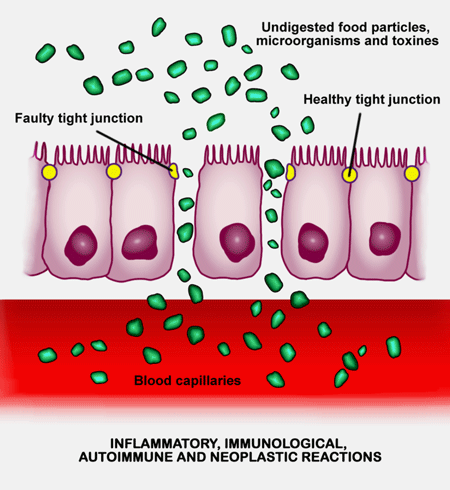
Long COVID, ME/CFS, FM all appear to feature increased gut permeability and tryptophan metabolism problems. (from Balena Blanca, Wikimedia Commons).
Just as in the severe COVID-19 patients, they found evidence (zonulin or tight junction permeability) that the gut walls of the long-COVID patients had become leaky. Increased beta-glucan levels indicated that gut bacteria had made their way into the bloodstream. The fact that people with more gut bacteria in their blood had more symptoms indicated that leaky gut was clearly contributing to long COVID! A correlation analysis suggested that inflammation was higher in these patients as well.
The Gist
- Symptom assessments just won’t cut it! So said the first presenter in the NIH’s RECOVER Review series. We need biomarkers – and plenty of them: mechanistic, diagnostic, prognostic, and above all “surrogate” biomarkers. (Who knew there were so many different kinds…)
- The HIV/AIDS field struggled for years with a lack of biomarkers. Only when the right biomarker was found did the treatment options for HIV/AIDS explode. Dr. Peluso predicted the same thing will happen for long COVID – it will just happen more quickly.
- Several different “mechanistic” biomarkers that are able to chart the severity of the illness are being investigated including inflammation, autoantibodies, coagulation, cortisol, microbial biomarkers in the gut, and exercise study results.
- Peluso predicted that more complex studies are on the way that will bring us closer to finding biomarkers.
- Dr. Walt – a renowned inventor – has invented a many, many times more sensitive way to find proteins, molecules, cytokines, etc. His early long COVID study found, to his surprise, evidence indicating that an actively replicating virus was still present in many people with long COVID but not in people who had recovered.
- His latest study has, thus far, not found such an incidence of an active virus in post-Omicron patients and it’s not clear why. We should know more about what’s happened soon.
- Dr. Abdel-Mohsen found evidence that a) leaky gut was present, b) that gut bacteria was making its way into the bloodstream, c) that it was causing inflammation, and d) most importantly of all, that it appeared to be interfering with the metabolism.
- It appeared that the coronavirus had interfered with tryptophan metabolism in the gut and gave rise to a host of metabolites that can trigger the NMDA receptor in the brain to produce glutamate which can produce neuroinflammation.
- Simply putting plasma from long COVID patients was enough to trigger inflammation – apparently because of the bacteria present – in cell cultures People with more neurological problems tended to have more symptoms and more gut bacteria in their blood.
- Finally, another study didn’t find evidence of endothelial dysfunction but did find evidence of arterial stiffness in long COVID.
- While the presenters didn’t mention it, with the exception of viral persistence, all these potential biomarkers have been found in ME/CFS/FM. Leaky gut is found, problems with tryptophan metabolism, and arterial stiffness have all been found – some over a decade ago – in these diseases as well.
- The research environment, however, has never been robust enough for these findings to reach “culmination” – to get to a point where they trigger studies that provide definitive results. Given the resources dedicated to long COVID those studies should occur and biomarkers should be found. Given the strong overlap between ME/CFS and long COVID, one has to ask why those biomarkers wouldn’t apply to ME/CFS as well.
Then came the really interesting part. Having increased inflammation was an outcome no one would desire, of course, but things potentially got quite a bit nastier when the researchers considered the metabolic implications of the altered floral bloom the coronavirus had triggered in the long-COVID patients’ guts.
Tryptophan may be an essential amino acid, but if it’s not broken down properly in the gut, bad things can happen. Toxic metabolites such as quinolinic acid that can affect brain functioning can build up. These can activate the NMDA receptor which, in turn, turns on the excitatory amino acid glutamate. (Think “brain on fire”). High glutamate levels can damage neurons and cause inflammation and have been associated with neurological diseases.
Three studies have found at least modestly increased quinolinic acid levels in long COVID, but the NMDA story doesn’t end there. Abdel-Mohsen found increased levels of other metabolites (e.g. S-sulfocysteine) that can turn on the NMDA receptors as well. Plus, the more neurological symptoms a person had, the higher levels of NMDA-associated metabolites they had.
Abdel-Mohsen, then, provided a potentially clear link between a virus-triggered gut flora alteration and neuroinflammation.
A theme showed up during these presentations – multiple large laboratories working together – and so it was with Abdel-Mohsen, who stated:
“we are working with many others in current and future studies to investigate both the diagnostic and prognostic potential as a biomarker or function as a mechanism of this marker of intestinal permeability, microbial translocation, and also host metabolite during PASC”.
It’s this mass of research groups that are working together on long COVID that’s going to make the difference. It’s not, after all, as if tryptophan issues and tryptophan metabolites haven’t shown up in ME/CFS and FM before. Interest in tryptophan in fibromyalgia dates back to 1986! A 1998 paper concluded that “The underlying mechanism (in FM) seems to be defective absorption of the precursor amino acid tryptophan from the gut”. Almost 20 years ago, Blankfield proposed both FM and ME/CFS were tryptophan-kynurenine pathway disorders.
More recent evidence suggests the same. A 2021 study, a 2022 study, a 2021 review, and others have explored problems with tryptophan metabolism in ME/CFS. Tryptophan metabolism is also the key pathway featured in Robert Phair’s Metabolic Trap hypothesis for ME/CFS. You could probably have substituted long COVD for ME/CFS in this paragraph from the 2022 study.
“Changes in tryptophan metabolites… in ME/CFS could both be compatible with anomalies in the sphere of energy metabolism. Overall, clinical traits together with serum biomarkers related to inflammation, intestine function, and tryptophan metabolism deserve to be further considered for the development of personalized medicine strategies for ME/CFS.”
So we see yet again an apparent overlap (leaky gut, tryptophan metabolism) in physiological problems in ME/CFS/FM and long COVID. The problem with ME/CFS/FM has been the scattershot nature of the field. Instead of large laboratories working together to track down the clues, ME/CFS and FM researchers’ efforts generally have consisted of mostly small studies that have slowly been building a case for, in this instance, tryptophan metabolism. They may have been right all along – in fact, this long-COVID study suggests they were – but they never gathered enough oomph to concentrate the field and give us the studies that would provide some definitive answers.
It’s this kind of scattershot, inefficient, and ultimately not very revealing approach that permeates so much disease research that caused the RECOVER Initiative to focus so heavily on building an infrastructure that can methodically collect data and test hypotheses, discard those that don’t work and move with on with increased focus with ones that do.
Dr. Grace McComsey – Blood Vessel Biomarker
Dr. McComsey emphasized that multiple biomarkers associated with different types of long COVID are probably going to show up and that the field needs stable, reproducible biomarkers that have been validated by multiple studies. Then she introduced a surprise.
Stiffened blood vessels that could impair blood flows have been found in long COVID, ME/CFS and FM.
Her studies did not find problems with endothelial functioning (whoops!) but did find large changes in arterial plasticity or stiffness (nice!). That’s actually a pretty nice finding for fibromyalgia and ME/CFS as multiple studies have found increased arterial stiffness in FM, in particular. Lynn Hodges’s fascinating New Zealand exercise study even found evidence that exercise increases arterial stiffness in ME/CFS. That increase in stiffness could make it difficult to get enough blood to the muscles during the recovery period.
Dr. McComsey then brought things full circle when she reported that several markers of inflammation, as well as markers of gut integrity, were associated with arterial stiffness in long COVID.
On a last note, she reported that people with low vitamin K status ended up with much more severe diseases than those with good vitamin K levels.
Conclusion

Treatments for HIV/AIDS exploded after the right biomarker was found. The same could happen for long COVID – and given the many overlaps – ME/CFS/FM as well.
All in all, the RECOVER Initiative provided a fascinating, as well as encouraging, review session. Viral persistence is a possibility for long COVID but nothing more than that at the moment. It was the gut and arterial stuff – and the continuing rather astonishing overlap that continues to show up between ME/CFS/FM and long COVID – that really stood out. ME/CFS/FM wasn’t mentioned at all, but it was like the elephant in the room that everyone either didn’t see or was ignoring.
Gut microbiome alterations, leaky gut, and arterial stiffness have been found in all three diseases – and some of the findings in ME/CFS/FM date back decades. The ME/CFS/FM fields have never had the resources, however, to bring those findings into the limelight and get them definitively studied. Long COVID, on the other hand, appears to have the resources to do that.
The inability of the small ME/CFS field to come up with any recognized biomarkers at all over the past 40 years has dramatically stunted the production of therapeutics. The HIV field was in the same fix for years until a surrogate biomarker was found – and the race for therapeutics was on. After that, treatment possibilities exploded.
Dr. Peluso believes the same thing will happen more quickly with long COVID, which brings up the question: “What about ME/CFS, FM, and similar diseases?”. Given the close physiological overlap between ME/CFS/FM and long COVID, my question is: why would a similar biomarker not apply to these diseases as well? Dr. Nath may be right – if you solve one of these mystery diseases, you’ll probably be well on the way to solving the others. Let’s hope so.
BIG (Little) Drive Update
Thirty K is a small number for most organizations but it’s a big number for us and we’ve just about reached it – thanks to the over 350 people who’ve helped keep Health Rising on the web for another year.

An explosion in therapeutics sure sounds like a good idea. If this review session is correct it could happen in long COVID (and if in long COVID why not ME/CFS/FM?)
Biomarkers have been whispered around the ME/CFS field for decades. Everyone knows we need a biomarker but this blog really laid out why – and explained why we may very well get one – not just for long COVID but possibly for all three diseases. That, in turn, could result in an explosion in therapeutics…
That’s what digging into this stuff revealed – and digging is what Health Rising does. If that enriches you please support us.






What if the biomarkers responsible were already known……. Herpesviruses 🤔
I have active HHV-6 and I’m in remission right now. So that could be a trigger but likely not the reason for ongoing symptoms. Sorry. I definitely believe there is a genetic component to this. It would explain why people have so many different symptoms from each other.
The genetic component could be a weakness of the immune system in keeping these viruses under control?
Gut bacterial profiles differ and the genes that they activate will differ
EDS. I’m certain of it. Tissue types. As far as I know ( which is not much I admit, ) there isn’t a comprehensive analysis of all collagen and tissue types . But eds is always somewhere close by. Every CFS person I know share eds features. Perhaps not enough for a full diagnoses, but I think that because the field hasn’t explored EDS properly
Has any medical research focused on environmental causes, such as chronic Lyme disease, the millions of environmental toxins we ingest and breathe daily? Functional/ holistic providers have been focusing on the microbiome and “leaky gut” for a long time.
I have hEDS .
What about the biomarkers idenfied by Dr Alain Moreau open med-Montreal. His study has identified micro rnas that are associated with those that have mecfs, different ones for those that have fibro and then the combo. From what I understand He plans to start testing as a diagnostic in Canada?
This biomarker is clearly not diagnostic yet, and it must be sensitive and specific enough to meet the criteria, which it is not.
This is a reply to Wayne, it does not seem to have been successful.
Can you include a link to the presentation? Thanks in advance.
Right
https://recovercovid.org/docs/R3.Seminar.17.11-8-22.pdf
https://www.youtube.com/watch?v=V-X3mNbHT1A
Thanks Cort for yet another great blog!!
I have the feeling that most of the research that is already out there contains most of the pieces of the puzzle needed to unravel the ME/CFS/FM/LC mysteries. “Old” research as you mentioned, that could not be followed up because of lack of money, combined with Long-COVID research and a pot of gold in the US to really get to the bottom of it will deliver.
There must have been COVID patients on the ICU already developing long-COVID. More than a year ago there were 180.000 papers on COVID; somewhere in there it might be hidden; it just has to be unearthed and combined with ME/CFS research.
Another thanks for your tip on weightlifting, a couple of blogs ago. It helped me with my stairs problem. I was panting, with heart pounding and head throbbing after just 13 steps. Now I do it a lot slower, one step at a time, lifting my weight, and I’m OK op top!! I’m a slow learner, I really needed your blog. Not my heart but my slow metabolism was the problem. Blood that is thick and sticky makes oxygen and nutrients not getting there in time?
Je suis interéssée par le conseil sur l’haltérophilie. Puis-je avoir le lien s’il vous plaît? Dès que je fais un effort, j’ai du mal à respirer. Le coeur est bon, les poumons sont bien. Je pense aussi que ça vient du métabolisme, car après mes séances d’ostéopathie, ma respiration est plus facile. I’ostéo travaille sur le nerf vague et sur les viscères. Il me dit que le sang stagne au niveau du ventre. Je dois manger de petites quantités par repas .
Grand merci à Cort, quel travail formidable il fait ! C’est magnifique. Bravo.
Et bonne fêtes de fin d’année à tous.
Two comments:
The tale of an actively replicating SARS-CoV-2 virus as the driver of PASC comes up again and again – unfortunately it is incompatible with the fact that S1-vaccination alone can cause exactly the same subtypes of PASC (POTS and ME/CFS). Either you believe in two distict routes to the same clinical presentation or you just scratch the persistence hypothesis. I´d go for the latter – especially as we all know how many viruses can trigger ME/CFS – and certainly the most plausible common determinator here is not persistence of the trigger but reactivation of preexisting (latent) viruses (which certainly can also influence permeability of the intestinal epithelial barrier, by the way).
Dr. Peluso believes that it is not valid to use “patient-reported outcomes” as core outcome measures for long COVID. This may be correct if all patients fulfilling the criteria for Long Covid or PCS are lumped together in a study. However, this may be entirely different if one studies Long Covid patients fulfilling strictly assessed ME/CFS criteria only. We have yet to see someone following this approach. It may be very fertile because from the start you´d have filtered out the many cases of protracted postviral reconvalescence which over time get better on their own and which plausibly have a distinct biopathological basis.
So viruses can alter your gut flora. So can antibiotics. This can lead to leaky gut.
So one must take probitiocs and also go to a Holistic Doctor to heal the leaky gut.
There are also supplements for inflammation to take while you have covid and CFS.
if any of you are in the NJ area, there is a HOLISTIC Doctor, IN -NETWORK (!), that treats covid and long-covid. Feel free to contact me via this site for his name and address. He is in central NJ, on the east side of NJ.
It will work to an extent. What we need are fecal transplants from a donor who has the bacteria we need and a way to ensure engraftment.
But since poop cant be patented, we are out of luck. Money trumps lives as usual.
Incidentally, viruses like EBV and Norovirus can damage neuromodulatory cells in the gut too. Maybe that is how they shift the microbiome? Or one of the ways? Who knows
I am in Atlantic Highlands NJ .Have been looking for a holistic doctor in central jersey.I would so appreciate the name and address of the doctor you mentioned.
The Dr. is:
Dr. Richard Menashe
15 South Main Street
Edison, NJ
PHONE: (732) 906-8866
The NJ Doctor is:
Dr. Richard Menashe
15 South Main Street
Edison, NJ
Phone: (732) 906-8866
Thank you for distilling all these reports for us Cort! It’s very helpful and it sure seems like a lot of progress has been made in the last several years! Praying for everyone that a breakthrough comes in 2023! Merry Christmas and a Happy, Healthier, New Year!
Cort, your write-up here is a wonderful gift to us patients this holiday season. Reading it gives me hope that things are really starting to move towards what I’ve been praying for for over 38 years: an effective treatment becoming available. As ever, thank you for all that you do.
Merry Christmas and Happy New Year to all!
Thanks Dan. I was really pleased to hear Pelsuo talk about how important biomarkers are and how the search is on! Of course, the remarkably similar physiological findings in these diseases is great to see. Let’s hope that keeps up. 38 years is enough!
Great write-up
I checked the video and I think your current text misses the fact that Walt analyzed two post-Omicron PASC datasets.
In the first, they found spike in 78% of cases (ie. more pre-Omicron).
In the second, they found spike much less often (no numbers).
To me, this sounds more like an issue with the batch of samples than anything clear about Omicron PASC.
Might be good to correct 🙂
I wasn’t aware that NMDA receptors were turned on in the illness. Agmatine Sulfate is like a scaled down ketamine with its ability to block nmda receptors. Might that hold promise? I know that dextromethorphan is suggested occasionally.
(…)that the spike protein persisted in 65% of the long-COVID patients(…)
Bad news. If you consider that the mRNA vaccine causes your own body cells to produce spikes ( for months) to produce antibodies. And this after every shot wow…
In my opinion you can get long covid after a covid infection but also after vaccination with Mrna.
That said, I don’t think this has anything to do with ME/CFS.
Or perhaps Memantine to block glutamate ….
Corrected web link for tryptophan/kynurenine pathway…
https://pubmed.ncbi.nlm.nih.gov/23922501/
Demeirleir was always ahead. He was testing kynurenic acid when I saw him many years ago….
I wonder if persistent HHV6 or EBV can also cause the leaky gut, and that leak is it related to the food reactions that some people with me/cfs experience. Beta glucans provoke the macrophages which is a word I have heard recently in relation to ME/CFS, something about them migrating into the brain was that it ?
There are so many news articles or research papers that have came out about these post viral diseases however this one stood out.
have you read the newer MS papers? They link EBV with a gene they are calling EBV-INDUCED GENE 2″. This gene, when expressed, results somehow in the body producing less interferon type 1, which is what me/cfs patients have i think. And some key species of clostridia produce a chemical that down regulates that gene…which I guess we do not have in our guts. That is the same bacteria they have identified in super donors that can treat autoimmune disease with their poop.
Linda, could you point me to where you read this? Interested but can’t find it with my current brain fog.
https://www.frontiersin.org/articles/10.3389/fped.2019.00059/full
oxysterols are what is produced by these particular clostridia species, that can down-regulate the gene triggered by EBV.
there are many papers, here is another one:
https://www.mdpi.com/2073-4409/11/22/3619
this gene may be one of many involved; it is over my brain fogged head
Super donors are rich in Clostridia XIVa and IV Clusters. These species produce oxysterols that down regulate EBV-induced Gene 2? I think. This gene is upregulated in MS and some ME/CFS patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4569432/
For what it’s worth, I have scoured Lyme disease groups and something I find interesting. Many people have reported remissions with antivirals, antibiotics and probiotics in combination. Some take hydroxychloroquine too.
hong kong is doing a long covid trial using fecal transplants
Cort,
If tryptophan is not metabolized properly and these metabolites cause big problems in the brain (20 years of research),
then does 5-HTP
(5-hydroxytryptophan) also create the same metabolic problem in the brain as tryptophan?
5-HTP is a chemical that the body makes from tryptophan. Many practitioners widely suggest using 5-HTP for sleep issues in ME/CFS/FM.
Is using 5-HTP detrimental to the tryptophan-kynurenine pathway? Maybe 5-HTP I’d helpful to this pathway?
Does anyone have information on this they can share? Thank you…and happy holidays.
https://pubmed.ncbi.nlm.nih.gov/23
922501/
Cathy, I take 5-HTP every day and it helps me a lot. If I take the capsules it hurts my gut so much, but I put the powder under my tongue twice a day, spit it out and rinse. It has helped my brain fog and muscle stiffness.
Biomarkers would be great, but what the article fails to address is the fact that AIDS was found to be caused by a single virus, HIV. We haven’t yet discovered a single virus responsible for ME/CFS. Still hoping for progress, but certainly not holding my breath by comparing it to AIDS research and treatment development.
But Lanetta – take away the different pathogen issue and we’re left with conditions that look almost identical otherwise. Aren’t these downstream overlaps (exercise studies, fatty acid issues, gut issues, arterial stiffness, herpesvirus reactivation, autoantibody production etc.)likely to be more important than whatever trigger there was. The fact that we’ve found commonalities in ME/CFS itself – despite its many different triggers – suggests that core problems are present.
Better treatments would be great! Because of the complexity of ME/CFS, I honestly just think it will be more difficult than the AIDS treatments were. I do hope I’m wrong, though. I’d especially like to see progress and development on the metabolic targets. And let me take this opportunity to say thank you for all the work you put into every article. Some days I love the details, other days “the gist of it” is all I’m able to absorb, so I appreciate that condensed version too.
Hi Cort,
we need the money, that is the problem. Long covid has a lot (was it 1.6 or 1.9 billion) from congres. HIV got money. And we suffer over decades with tiny small research projects because of lack of money or research projects that simply can not be done because of zero money. Just like FM, etc If we had the amount of money long covid has gotten we would not be ill anymore or not so ill at least… only an overlap here and there that you notice, where no big research with big money is done for us, will not help us. maybe i misunderstand but i can only count the amount of money for long covid and for ME,cfs, FM, etc
Cort,
If tryptophan is not metabolized properly and these metabolites cause big problems in the brain (20 years of research), then does 5-hydroxytryptophan (5-HTP) also create the same metabolic problems in the brain as tryptophan?
5-HTP is a chemical the body makes from tryptophan.
Many practitioners highly recommend using 5-HTP in their protocols for ME/CFS/FM for sleep issues.
Is 5-HTP harmful in the tryptophan-kynurenine pathway?
Or maybe 5-HTP is helpful to the tryptophan-kynurenine pathway?
Does anyone have information on 5-HTP and how it affects the tryptophan-kynurenine pathway…good or bad? Asking since 5-HTP is suggested in treatment. Will 5-HTP block pathway?
https://pubmed.ncbi.nlm.nih.gov/23922501
Thank you…and happy holidays.
Es curioso todo esto me lleva a la enfermedad llamada SIBO. En recientes estudios se ha visto que la prevalencia de la misma en pacientes con SFC/EM es del 80-90%.
La cuestión es si SIBO es la causa o una consecuencia de padecer SFC/EM
Interesting Fran. Maybe SIBO stops tryptophan from processing properly. I agree it us likely ME/CFS persons have SIBO too.
Hola Cathy
Se estima que hasta un 35% de la población general puede padecer sobrecrecimiento bacteriano, pudiendo aumentar la prevalencia hasta el 80-90% en pacientes con Síndrome del Intestino Irritable o con Síndrome de la Fatiga Crónica
En este estudio, el Dr. W. Ian Lipkin y sus colegas de la Universidad de Columbia han comparado un grupo de enfermos de EM/SFC con individuos sanos. Algunos sujetos del grupo de EM/SFC ya habían sido además diagnosticados con el síndrome del intestino irritable (un trastorno funcional gastrointestinal).
Como bien dices, el aminoácido triptófano no se absorbe bien en el intestino Delgado y una de las causas puede ser el SIBO.
Al no absorber triptófano, no se fabrica serotonina ni melatonina
Esto podría inducir a estados de ansiedad, estrés, depresión, insomnio.
Loving the explanation of four types of biomarkers.
Also it seems like a good thing that an HIV researcher is involved, so that any relevant knowledge transfer can happen from that direction.
Not pleased about ME/CFS being “the elephant in the room” & seconding Herbert’s comment on patient reported outcomes. It might be possible to do ME/CFS criteria assessments on existing patient cohorts and stratify?
Thank you Fran and Ann 🙂
A lot of consistencies with Dr. Galland’s research from a year and a half ago. Re: gut dysbiosis, fungal translocation, leaky gut, tryptophan, etc:
https://drgalland.com/coronavirus-protection-protocol/
As a Longhauler, the protocols I have had the most success with in terms of symptoms are Leo Galland’s, Tom Bunker’s, and the FLCCC. There is a good deal of overlap amongst the three.
Hey Cort, I am just reading or re-reading some of your older articles (with the brain fog I usually have to read everything over and over until it sticks) and now I’ve stumbled over the name Dr. David Walt. I have never heard of him elsewhere but it seems his research could open quite a few doors. Apart from his teams work on viral reactivation you state he has a technique that’s 1,000 to 10,000 x’s more precise than standard ELISA techniques. Wow! If that’s true than that could really make a difference in praticular for the GCPR AAB theory which always seems to be on shaky legs with noisey measurements. Do you think it would be worth it to dedicate an article to his teams research?