
A biomarker may have been found that could facilitate the production of treatment trials.
The fact that two NIH-funded chronic fatigue syndrome (ME/CFS) gut studies were published on the same day wasn’t an accident – an effort was clearly made to highlight their findings. Indeed, they were accompanied by an NIH blog, “Studies find that microbiome changes may be a signature for ME/CFS“, suggesting that their results – which mirrored each other – indicated that a biomarker may have been found.
Vicky Whittemore Ph.D., program director for the NIH’s National Institute of Neurological Disorders and Stroke (NINDS) said:
“These findings provide unique insights into the role the microbiome plays in the disease and suggest that certain differences in gut microbes could serve as biomarkers for ME/CFS,”
Given how conservative the NIH is, any talk about a biomarker is noteworthy. The NIH, though, wasn’t exactly out on the skinny branches with that possibility. The studies were big enough (>200 people per study) and rigorous enough so that any common findings would automatically bring up talk of a biomarker.
It appears that researchers are starting to zero in on some core and reproducible findings in the gut in ME/CFS. That’s a pretty good trick given how many bacterial species researchers plow through in these studies.
The key finding from both studies concerned low relative abundances of butyrate-producing bacteria, with the Guo study highlighting low levels of several bacterial species (Faecalibacterium prausnitzii), Eubacterium rectale), and the Xiong study reporting that the gut disruption was much worse in the shorter duration (<4 years) compared to longer duration patients (>10 years) who had worsened metabolic issues.
The Lipkin Group Study
Several hurdles the Guo/Williams study (“Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS“) from the Lipkin group had to surmount made it clear that its results were designed to stand the test of time. Instead of studying a single, geographically isolated study population, the participants came from across the U.S. Instead of one sample taken at one point in time, the participants provided four samples spread across a year. Getting positive results from different groups spread over a long time period signaled stability; i.e., these are results we should be able to count on.
Just about every major gut assessment (gut microbiome diversity, microbiome abundances, functional pathways, and interactions) was altered in the ME/CFS patients vs the controls. The low abundance of two common bacterial species that figure prominently in butyrate production (Faecalibacterium prausnitzii, Eubacterium rectale) stood out. Butyrate is an important anti-inflammatory that helps gut health in multiple ways.
Multiple tests (functional metagenomics, qPCR, metabolomics analysis of fecal short-chain fatty acids) confirmed on the ground level what the microbiome analysis suggested had happened: ME/CFS patients’ guts are plagued by a reduction in butyrate synthesis. Several analyses also showed that in every case, if you have ME/CFS and IBS, gut issues at the pathophysiological level were more severe.
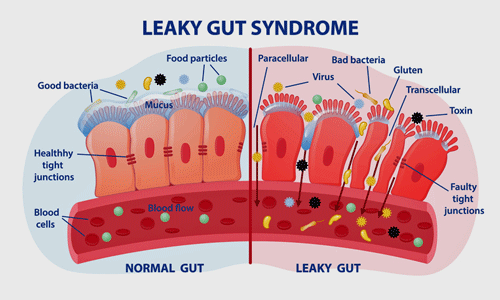
Reduced butyrate levels could lead to leaky gut syndrome and inflammation in the body and brain.
The more the Lipkin group dug into butyrate production, the more they found. Four pathways of bacterial butyrate production exist: the acetyl-CoA pathway, the glutarate pathway, the lysine pathway, and the 4-aminobutyrate pathway (Figure 3C). While three of the pathways appeared to be intact, a metagenomic analysis found what appeared to be a remarkable disruption in the acetyl-CoA pathway, with deficiencies found in virtually every gene in that pathway. Finding such a narrow zone of disruption is encouraging as it provides an easier target for potential treatments.
Low butyrate levels could impact everything from colon health to inflammation, T-cell regulation, and integrity of the gut lining. The low acetate levels that were also found could potentially feed right into the low butyrate findings as acetate is a preferred food for butyrate-producing bacteria.
The low butyrate levels could also be contributing to the increased “bacterial load” found in the ME/CFS patients’ guts. Since butyrate has antibacterial properties, the low butyrate levels may be impeding the ability to control bacterial growth.
F. prausnitizii stood out because it’s been found reduced in at least one fibromyalgia study and three ME/CFS studies. This odd bacterium, which does not produce spores and moves around very little, makes up a full 5% of the bacteria found in our guts. Through its fermentation of dietary fiber, F. prausnitizi produces butyrate and other short-chain fatty acids, as well as an important anti-inflammatory product. It’s been called “a potential biosensor of human health”.
The findings played out on a symptom level as well: the less F. prausnitzii a person had, the more fatigued they were. Interestingly, reduced levels of F prausnitizii have been associated with fatigue in inflammatory bowel disease.
We’ve seen evidence of “rewirings” of the immune system in ME/CFS before, and now these researchers reported that an “extensive rewiring” of the microbiome had occurred as well. Again highlighting its potential importance, in what appeared to constitute a kind of ecosystem shift, F. prausnitzii appeared to play a bigger role in the ME/CFS microbiome than in healthy controls.
The Gist
- This is new… Two NIH-funded research center studies were published simultaneously and were accompanied by an NIH blog. There was a reason for all this synchronicity – the two large studies confirmed each other’s findings – leading the NIH to publish a blog suggesting that a biomarker may have been found.
- The biomarker concerns low levels of the anti-inflammatory and gut-protective substance butyrate in the guts of ME/CFS patients.
- Part 1 of the 2-part blog series concerns a paper from the Lipkin group. With its large number (>200) of geographically diverse patients, and its multiple sampling times, this study was designed to produce results you can take to the bank.
- Just about every major gut assessment (gut microbiome diversity, microbiome abundances, functional pathways, and interactions) were found to be altered in the ME/CFS patients vs the controls.
- The study assessed butyrate production in multiple ways – via microbiome analysis, aPCR, and metabolomics – and in every case found evidence of low butyrate.
- The low abundance of two common bacterial species that figure prominently in butyrate production (Faecalibacterium prausnitzii, Eubacterium rectale) stood out. F. prausnitizii has been found reduced in at least one fibromyalgia study and three ME/CFS studies. Called “a potential biosensor of human health”, it produces butyrate and other short-chain fatty acids, as well as an important anti-inflammatory product.
- An “extensive rewiring” of the gut microbiome that prominently featured F. prausnitzii was also found. Plus, a metagenomic analysis found what appeared to be a remarkable disruption in the acetyl-CoA pathway of butyrate production, with deficiencies found in virtually every gene in that pathway.
- Other evidence of possibly increased lactate production and mucin (the protective substance that clothes the gut walls) degradation was found.
- Because physical activity increases butyrate levels, the inability to engage in much physical activity could play a role in reduced butyrate levels in ME/CFS. The second paper, though, which found more gut disruption in shorter duration patients and more metabolic problems in longer duration – and presumably less active – ME/CFS patients argues against that conclusion. Still, activity levels will need to be taken into account in future studies.
- Even if low activity levels are impacting butyrate production, the low butyrate levels may need to be addressed before people with ME/CFS can exercise again. This is because butyrate protects against leaky gut and one study showed that exercise dramatically increased leaky gut and inflammation in ME/CFS.
- We still don’t know if the gut findings are secondary to the disease or play a more primary role. Similar gut findings in several ME/CFS studies, though, are clearly opening the possibility of treatment trials and other studies that perturb the gut to see if symptoms improve.
Another factor might be in play, though. Animal models and some human studies have found that physical activity results in higher levels of butyrate producers like F. prausnitzii’. That suggests that the reduced butyrate production found in ME/CFS, could, at least in part, be due to the dramatically reduced physical activity the disease produces.
On that note, it’s interesting that longer-duration ME/CFS patients in the Oh study who have more severe metabolic issues – and are presumably less active – didn’t display as severe butyrate depletion (or other gut issues) as the shorter-duration patients in the study. That suggests that if reduced activity plays a role in lower butyrate production in ME/CFS, it may play only a partial role. It’s clear, though, that activity levels will need to be taken into account at some point in these studies.
If some of these gut changes are caused by low activity levels, they would still need to be dealt with as reduced butyrate production would surely contribute to the leaky gut and exercise problems found in ME/CFS. We saw dramatic evidence of this when exercise dramatically increased leaky gut and inflammation in ME/CFS compared to healthy controls. Even if the reduced butyrate production is the result of reduced activity, that problem will probably need to be resolved before people with ME/CFS can begin to engage in strenuous exercise again.
It was good to see F. prausnitzii levels correlated with fatigue, but we still don’t know the real impact the gut issues have on symptoms. We don’t know if they are a secondary outcome of the disease or play a primary role. It will take treatment trials and other studies that perturb the gut to see if symptoms improve to answer that question. The fact that a clear abnormality (Iow butyrate levels) has shown up across multiple studies suggests that researchers are willing to take that next step and start perturbing the gut in ME/CFS to see what happens.
- Coming up next – the fascinating Oh gut paper suggests how ME/CFS may have gotten started





Interesting. Plenty of studies on how to try and increase levels of F. prausnitizi inulin and lactulose are 2 I’ve seen but lactulose is laxative. I already take inulin, still have ME though.
What about taking Butyrate as a supplement?
You can take butyrate as a supplement. Here is one that I like:
https://healthygut.com/product/tributyrin-x/
The information lower on the page says almost the same thing that Cort has said here. It even has the same diagram.
That’s what we’re going to try on my husband, along with increasing fermented foods.
Inulin has caused diarrhoea for me and taking butyrate caused heartburn. I had FMT which has brought a big improvement but not a cure.
My daughter has ME/CFS. She has suffered extremely painful stomach attacks. So there does seem to be a link between these stomach attacks and her disease.
Has your daughter also been tested on stomach ulcer?
We saw a Dr at the time.
When I first fell ill in the early 2000s, i had regular excruciating stomach pain. Really so painful that i wanted to cry. It has subsided significantly over the years. I still occasionally get it, but perhaps only a few times a year. I’m still ill, but mildly impacted. All the other symptoms, pain, fatigue, twitching muscles, memory issues persist.
Ted –
I also had these intense stomach attacks. They were brought on by specific foods, most of the time.
Many years later, I finally had full-blown pellagra.
Reading through the older medical literature, from when pellagra was endemic, this mysterious ‘gastritis’ is described, as preceding the severe state by years.
It also figures in the porphyrias, specially the acquired one. I don’t see a difference. In fact, at some point certain porphyrins were measured to be present in excess in the urine when the pellagrin was symptomatic, and normalized when they were cured. From what I recall, they were the same porphyrins as the ones measured in that acquired porphyria. This of course, has been lost in contemporary medical knowledge/practice. I think its the same disease.
Porphyria is treated by diet – making sure to eat plenty of carbs throughout the day.
Looking back, the foods that triggered my mysterious stomach attacks were high in leucine. This is a driving factor in pellagra. See David A bender if interested.
Pellagra was cured by changing the diet – their protein intake was low (though high in leucine from the corn/millet/whatever) so an imbalance of amino acids) And switching from grains to fruits, etc.
A comment to you misplaced a few lines lower.
Thanks M
I reckon that if you are not in a very hot, strong sun climate, the pellagra might not present with the 3Ds, as it is known today. That’s why I find the older texts useful, because they describe a full picture, and the preceding years. You xan find them on the free internet archive.
Worth a try taking some niacinamide, 50-100mg a few times a day, and see what changes that brings on about.
Usually having some carbs with it – or follow your cravings.
Well, Cort, this report is surely interesting. I mentioned to you years ago about the gut and I think it was u of NC interested. I know that if this disease had been taken seriously, we would not still be in bed. Even if no answers were available we would have been better off just having doctors, our family, etc believe us. We have fought disbelief for decades. Being ignored or labeled hypochondriac or depressed has made us depressed for sure. I do thank the cosmos for researchers like dr. Lipkin and others who are trying. I do not forgive the NIH especially for pushing us continually to the back of the line if we were even in line. I wonder if Fauci himself as head of his department stopped progress also? Even if doctors believe it’s real now don’t know what to do. My doc just says..yes, it’s a hard one. Since the Sackler family ruined opioid help, it’s obvious the feds now are focusing of the benzos. As we know, the sympathetic nervous system is on go which is sending many of us on the adrenaline trip which is not my cup of tea. Very disturbing. Benzos can help but hard to get now. The beta blockers help but lower the heart rate for some to much. My life and I’m sure others has been so unproductive and sad for so long now. 36 years for me. I would so love to find my joy of life again. Love to all of us, Javen
Javen morell; In reading this article and the blog posts, I am especially drawn to your writing. It’s 42 years now since my life fell apart with Fibro and then CFS/ME. Every night I’ve gone to bed hoping the next day would be better. Essentially, it never happens, but I still hope for that. I deeply appreciate your words: “My life….has been so unproductive and sad for so long now..I would so love to find my joy of life again. Love to all of us…” Thanks for your insight and compassion – I wish primary care doctors would follow Fibro and CFS/Me and stop literally adding “insult” to injury. We have all endured enough for long enough. Hoping you (and all of us) make it through! “Be well!”
Dear Connie, I appreciate your comments. Love and we will continue to hope. Javen ….. so appreciative of Cort and this blog.
There are a lot of articles online regarding the dearth of f. prausnitzii in the gut biome of the obese, as well as those with digestive disorders such as crohns, etc. There are also numerous articles on methods to increase f.prausnitzii via diet, but no indication there is a supplement or probiotic that includes this in the formula. Are you aware of one, and who makes it?
Further, I doubt this is the only answer to ME/CFS. The dietary recommendations to increase f.prausnitzii include various roughage, fresh fruits and vegetables, beans, a mediterranean diet; these suggestions are ones that are currently used by many ME/CFS sufferers in our search for good health. One would think that if it was this simple we would all be running around, the picture of health. Sadly, not the case.
I am sure many of us would love to add this to our toolkit, it would be a great help if you could tell us where a supplement is available, as relying on dietary food intake to increase f. prausnitzii is clearly not working.
I haven’t looked recently but if memory serves F. prausnitzii is difficult to develop as a probiotic and none are available. Maybe someone knows better. I don’t think the gut is the only answer. Could fixing the microbiome help? I would think so. The next blog in this series suggests that even if it started out in the gut there’s more in play…
There are PREbioticts available that claim to increase F. prau. I know of two brands Livaux and Actazin, both made from kiwifruit.
This butyrate supplement is a “post biotic” as it explains on this page:
https://healthygut.com/product/tributyrin-x/
It also explains how the problems of delivery have been solved.
Have you used this? Has it helped your gut? Thanks.
A gut microbiome analysis I did in 2017 showed f. prausnitzii was absent from my gut. I’ve been researching f. prau and trying everything that had some chance of helping it since then and nothing has helped (not that I’ve redone the microbiome analysis). Because f. prau is anaerobic, it is very fragile outside the gut and can’t be taken in a normal capsule, pill, or liquid. There are some companies/researchers trying to come up with a form of encapsulation that will keep it viable, but it’s a way off being available last I heard. The other problem is that while f. prau is very abundant and common in normal human guts, it depends on several other species such as e. coli and bacteroides thetaiotaomicron. Those species are also absent form my gut, and there have been studies showing that e coli is absent or much diminished in CFS guts generally. Basically, for now there doesn’t seem to be much you can do that is likely to improve f. prau status (provided you’re already doing the obvious things like eating whole foods, sleep as well as possible, avoiding stress, exercising as able, etc.). E. coli supplements are worth a try though – seem to help some CFSers, certainly do help a lot people with Crohn’s.
I would be interested to know if any of you have developed PVCs and PACs. My humble opinion tells me it’s the overactivity of the sympathetic nervous system. Very uncomfortable feelings. The beta blocker retards them and the benzos also but the beta blockers lower heart rate to low for me personally and doctors are shying away from the benzos. I can breath deep and try to relax till the cows come home but that doesn’t work. Anyone else? Sincerely, Javen
Hi Javen yes I have developed pvcs and now have recurrent myocarditis which I am fairly sure are related to cfs. The cardiac symptoms increase when I have a bout of PEM. Myocarditis is often caused by viral infections and I suspect my herpes/Epstein Barr infection that triggered cfs 12 years ago is now affecting my heart. I haven’t seen anything yet in the health rising discussions that mention something like this so – anybody? Cort?
I have had PVCs and PACs since I was 20. (I’m 70 now.). I have had ME/CFS and Fibro for 45 years. But I was diagnosed with a mitral valve prolapse at 20 which causes those and was diagnosed with A-Fib ten years ago. Who knows what’s from what? A triple whammy, perhaps. I do take medicine to control the irregular heartbeat, but I still get the PVCs. And yes, it gets worse with PEM.
My gut is my biggest problem (other than I can’t walk anymore). I have a terrible digestive system with IBS-D and -C. It’s a daily struggle, painful, and uncomfortable… I would give my teeth to have a normal stomach and bowel. There is very little that I eat anymore. I can’t handle fermented foods. I drink a lot of water and use a probiotic (Physician’s Choice Women’s) and Benefiber, different types of Magnesium when needed, and Rx Lomotol when needed. I also take dicyclomine. It’s an unpredictable merry-go-round. My gut doctor clearly doesn’t understand how bad it is. He tells me that Benefiber every day cures it all. Well, no. I’m hoping this research continues and delivers some results that we can all use.
Thanks, Cort, for another great article.
thanks Kristine
Javen, You can retrain your autonomic nervous system. That is; train the sympathetic part back to normal . Methods include: coherent breathing, biofeedback, and retraining the limbic brain. Retraining takes daily practice for several months , but is totally safe with no drug side-effects. Nansy
Nansy, I’ve tried breathing but where to learn retraining the limbic brain? Have you done this? Were you having the same problems with the sympathetic nervous system also? Javen
Javen, I am using the Dynamic Neural Retraining System by Annie Hopper to retrain my brain. My ANS has been stuck in sympathetic mode for 60 years so it is taking a long time. The breathing needs to be done using the coherent method well documented by two docs. Their method is explained at Breath-Body-Mind.com. Nansy
I have PACs. They did a full cardiac workup and found no problems. They are scary but I’ve had fewer of them the last few months than before.
Ann 1 have you tried the Tributyrin-X supplement you men above? If so have you noticed any improvement in symptoms? 🙏
Hi Javen, I have PACs that increase with exertion. I’ve had ME/CFS/FM and dysautonomia for at least 30 years. I appreciate your posts and this website so much! It gives me hope.
Thanks for replying, Pat. Cort and this blog gives me hope also. Love, javen
“Since butyrate has antibacterial properties, the low butyrate levels may be impeding the ability to control bacterial growth.”
Makes me wonder if this could explain why patients with me/cfs seem more prone to getting lyme disease bacteria and some getting it particularly severely, needing to take big steps to recover.
Another what came first, the chicken or the egg?!? Some were active and athletic who’ve contracted CFS. I was not athletic but plenty active for 20 years with both CFS and Fibromyalgia until both became severe for me for no apparent reason. I believe these are autoimmune caused by virus reactivation going haywire in our bodies causing all the various possible symptoms, including these gut issues/imbalances. I can only hope they get it figured out so we can have some sort of quality of life again before we expire. Thanks for all the reporting and updates Cort. I have no idea how you’re able to do it.
One of the really interesting things I learned this past year was the dramatic impact that viruses can have on the gut. I had no idea that was happening.
Has anyone been known to have mold issues impact the gut in ME/CFS?
I agree Tracey. I’ve had ME/CFS for 35yrs. Since 2014 seemed to be getting worse. However just started taking Quercetin to reduce uric acid and i seem much improved much more clear headed but strangly still not able to retain info. Also seem a little less fatigued. I had a serious bowel overgrowth of bad guys, which has been treated by my naturapath for 4 months now so that is probably contributing too. I have to plan days out so my socialising is very limited. As you say it wouldbe nice to get a breakthrough before we expire.
The “chicken or egg” I have been curious about is whether covid causes “long covid,” or if covid is merely a “trigger” for a latent virus (i.e. epstein barr) that results in ME/CFS. I haven’t been able to find anyone producing solid evidence for either theory.
Hi Brian, I think you are correct, but I don’t think EBV is the cause. 90% of the population has EBV at some time usually in childhood. I believe HHV6A is the cause. You can’t get any statistics on HHV6A prevalence, because testing if not widely available.
This brings me to what I consider to be the “proof of an infectious agent as the cause of ME/CFS”. In most countries, you cannot donate blood if you have ME/CFS. https://me-pedia.org/wiki/Blood_donation
Many Lyme docs believe undiagnosed tick borne disease to be an underlying cause of ME/CFS. So as Betty mentions, there may be different infectious agents causing ME//CFS, yet you have to wonder why only some people get ME/CFS symptoms and not others exposed to the same bacteria or viruses?
We’ll be using Tributyrin-X as part of our RemissionBiome.org self-experiment.
https://healthygut.com/product/tributyrin-x/
A blog on Tess’s exciting Remission Biome project is coming up shortly 🙂
Will be interesting to hear how this fares.
Please keep us posted on how this goes. Do you know of anyone having success with this supplement lowering their ME/CFS symptoms? Thank you!
Firstly, thank you Cort for your laudable efforts in keeping everyone informed and updated
Gut bacteria is such a complex interplay of viruses, bacteria etc that isolating one such (prausnitzii) is probably too simplistic.
There is an ever raging war of good and bad bacteria ..or should I say dominant and subservient bacteria… just changing your location for a short time, or going bush for a walk can change the composition.
Great that for the investigation they sampled participants a few times during a year but ?were the participants homebound? Or limited in range in some way?
Perhaps the degradation of praunitzii is due to the dominance of some other species and no matter how you try to increase prausnitzii the action needed is actually to quell OTHER species. And that could be different for everyone.
I am pleased they are at least going this route to see what can be done via the gut cause I do believe it is hugely relevant to health and outcomes.
I am hoping the larger investment in research (with long covid in play) will throw some light in the ME/CFS arena.
Thank you, Cort, for taking two significant papers and not only interpreting them but also linking them to previous research, to articles you have written in the past, and to relevant research in other diseases. You are truly amazing and the work you do is staggering in its complexity and value.
Thanks Di! The next paper is fascinating! More twists to come 🙂
Dr. Mercola recommends taking Lactobacillus Reutiri in general to support butyrate levels in the gut. I started doing that recently. I’m gratified to see researchers showing more interest in gut health. Only good will come from that. 30 years ago when I searched in the National Library of Medicine, I found very little interest in nutritional subjects in the American research landscape. At the time I was looking into for nutritional protocols in order to push my fibromylagia into remission. The solution for me ended-up being an extended fast. But since then I’ve learned so much about an optimal diet from Dr. Steven Gundry and me and my family’s health has much improved on his low-lectin diet. I’m actually convinced it can improve or erase every chronic illness. My husband pushed his chronic sleep disorder into remission in just two months. He did not have ME/CFS but he’s been waking up 4x a night for 15 years so his healing was impressive.
Has l. Reuteri helped you?
I don’t think I have taken it long enough to really know.
All that from the Gundry diet! Impressive….I need to check that out given my frequent wakenings and short sleeps.
It’s a radical shift from eating the all-American diet and I think we could all be healthier if we even complied with it half-way. I do have to shop at an Asian market to find many of the vegetables and I also order some foods and products online but Dr. Gundy is very generous and gives such wise counsel and delicious recipes that have helped us immensely. I also follow Mary Ruddick, a young nutritionist who pushed her severe case of POTS/dysautonomia into remission after a year on the GAPS diet (plus brain retraining and lifestyle modification.) She does avoids multi-tasking, excessive media intake, practices meditation and keeps a gratefulness journal. The brain and gut dictate so much of our health and she covers a lot of the bases in her protocol. The Weston A. Price Foundation is a great resource as well as https://isom.ca/article/gaps-nutritional-protocol-how-healing-the-gut-removes-the-basis-for-all-chronic-diseases/.
Hi Maureen,
Very interesting commentary on the improvements you and yours have had.
What type of extended fast were you on and for how long?
My brother did an extended water only fast for nearly 40 days which shrunk a cyst in his brain that was giving him migraines and ocular changes. It resolved the problem for 13 years! I can’t remember the name of the book he read which he used to guide him. I’m not recommending this! Im just relaying info and curious to see if you did anything similar.
The cyst has returned and, now pushing 70, he’s unable to do another long fast.
I would consider doing a long fast given his past experience and your experience.
Any background on your fast and books/articles you read that convinced you to try it I hope you would share.
Thanks so much!
Kit
Hi Cort, I found this article very, very interesting because as well as having been diagnosed with Chronic Fatigue back in 2006, I was diagnosed with Crohn’s Disease in 1987 when I was in my twenties.
My Crohn’s disease is definitely genetic because my dad and sister both have Ankylosing spondylitis which is a related disease, but neither have ever suffered with inflammatory bowel disorders.
I took a look at how I could up my butyrate and saw that high protein, high fat and low carb diets have been shown to interfere with butyrate production and that reminds me how badly I did on the Keto Diet (High Fat) I tried last year…it was a disaster, I came out in rashes, had gastro disturbances and my chronic fatigue worsened. I have subsequently been on a medium to high carb diet, low fat and moderate protein and have noticed huge improvements in all areas.
Your article has justified my current view on my Crohn’s/Chronic Fatigue dietary position and I am very happy about that !
” In short, swapping out complex carbohydrates and whole plant foods for a diet dominated by animal protein and fats can have a negative impact on your bacteria’s ability to fulfill their destiny in your journey to health and well-being. Vegetables are your friends, readers. Fruit and whole grains too. Not to mention fermented foods, just not right now in this butyrate scenario”.
(https://atlasbiomed.com/blog/what-is-butyrate)
I hope this info might help others with similar problems as myself and again, thank you for the work you put in to keep us informed Cort.
Thanks Lynn for sharing that. I too did not improve on a keto diet. Interesting that a very different diet (medium to high carb diet, low fat and moderate protein) helped so much 🙂 . I agree – vegetables are our friends. 🙂
I had the same experience with keto (+ low histamine/oxalate/lectin) diet – I got steadily worse. As soon as I went back to a fairly “normal” but healthy diet with carbs, I bounced back immediately (still generally fatigued but able to do things). I have tried so many different protocols to try and improve my gut health over the years, but instead have progressively declined with more and more food sensitivities (and general gut problems).
I personally think everyone should get a GI Map done to understand what’s going on with their gut. I had one done by Diagnostic Solutions Laboratory (in Alpharetta, GA) and it showed up some interesting results. While my F. Prausnitzii is within the normal range (close to the low end though), I was completely missing Akkermansia Muciniphila (important bio-protective layer against leaky gut), low Enterobacter spp (due to leaky gut), very high Zonulin (indicator of leaky gut) and high counts of dysbiotic/overgrowth bacteria (Streptococcus, Enterococcus, Bacillus), and moderately high H. Pylori.
I am now seeing a gastroenterologist who is recommending a full FMT treatment. He looked at my GI map and basically said every time I eat my gut will be producing toxins that go straight into my bloodstream, causing systemic inflammation. He said that after a certain point your gut microbiome cannot recover no matter what diet and pre/pro biotics you take, and he has had a lot of success with FMT treatments for fatigue and IBS.
The FMT protocol is:
– 14 days of antibiotics and antifungals to kill as much of my existing microbiome (esp. dysbiotics).
– Bowel “flush” (from the chemist) the night prior.
– Initial transplant via colonoscopy to ensure it gets as high in the bowel as possible.
– 9 days of specially prepared enemas (from up to 3 different donors – all strictly screened).
– Special diet and precautions for a month following to help nurture my new biome.
I’m booked in for next month, so will report back here afterwards. I’m not expecting miracles, but believe a good gut microbiome will be one of the pillars for getting back to a somewhat normal life (despite having ME/CFS). Fingers crossed.
Fascinating Darren – I had never heard of a GI map. Be sure to check out the 3rd blog in the series where two women with ME/CFS are attempting a massive gut reset in an attempt to regain their health. What you’re doing reminded me of them 🙂
You can find it here – https://www.healthrising.org/blog/2023/02/16/remission-biome-gut-reset-chronic-fatigue-syndrome/
Time Health in the UK sell a product called ButyraGen that claims to be a ‘Direct Butyrate Generator’. I take it alongside a high CFU broad-strain probiotic and the combination definitely helps my gastrointestinal symptoms. Obviously I’ve no way of knowing whether it’s increasing levels of butyrate. Most of my other ME/CFS symptoms aren’t improved.
They’re working on F. Prausnitzii supplements: https://www.biogaia.com/pressrelease/metabogen-has-reached-a-new-crucial-milestone-in-its-development-of-novel-probiotic-products/
Great to hear! With all the attention the gut is getting I would be shocked if the probiotics we have right now are just the tip of the iceberg of what we’ll get over time. I can only imagine that they will get much more powerful.
Thanks for letting us know 🙂
Now that researchers are thinking MS is a gut-related disease, and MS may be a sister illness of ME/CFS, what are the studies in MS patients revealing about their butyrate? A quick glean suggests there is some role/connection in MS with butyrate.
Hello, I have ME/CFS and my sister has MS, so yes I believe MS is a sister disease 😉
It’s important to note that intestinal SFCA derived acetate is a significant contribution to the bodies overall energy supply with some estimates as high as 15%, this is especially important in people with chronic fatigue syndrome, where longer chain fatty acid metabolism is impaired on a cellular level. An interesting sidenote, is that a single episode of sleep apnea reduces intestinal butyrate production by a whopping 50%. This may be relevant to MECFS do to the common findings of sleep disruption. (this also has a surprisingly strong next day affect on blood pressure, causing a mild systemic vasoconstriction that raises blood pressure by after 10 points. I became interested in this years ago after I noticed a dramatic difference in brain fog, and overall feelings of wellness, depending on how my poop smelled. When my poop did not smell at all, I experienced more severe symptoms. When my poop smelled a certain way, the stronger that smell the easier it was for me to think and function that day. I try to adjust my diet to maintain that smell. Fun fact: butyrate is what they add to natural gas to make it smell, otherwise it is odorless.
Thanks Brian,
Acetate was like a side note but it looked like it had the potential to be a really big player given its role in producing butyrate. It’ll be interesting to see how the acetate connection develops. Maybe a big part of this is actually acetate (???)
If sleep apnea is doing that I imagine that multiple awakenings and fragmented sleep is as well. It’s so interesting how all this stuff starts to fit together.
Does anyone know what the results were of the FMT study done in Norway? Also which method was used for FMT? Did they ever publish results?
if you do mean this study, as i understand it. it is only completed in 2026. damn… link below.. but i know they are doing in France also a FMT study but forgot where. Also i wondered with the NIH studys that there was no gender differentiation. but i am verry ill and maybe it is not necesaerry for those studys. but like in the Norway study, they matched gender. And they are beginning more and more in studys to split the genders up. do you understand it from the NIH studys?
Also i saw long time ago a webinar where a women got 15 FMT’s and got “better” but everytime relapsed. I wonder why. If possible for me, i would try, even with the risks.
hope it is the study you named:
https://clinicaltrials.gov/ct2/show/NCT03691987
Yes that is the Norwegian study I was referring to. I have also read about MECFS patients who did not have long lasting benefit from FMT. I suspect that the GI immune system in MECFS patients has been altered and that is driving microbiome changes. Dr Unatmaz who is an immunologist at Jackson labs is apparently studying this effect.
also as i understand it, the FMT study in Norway is only a 1 time transplant and that i would have hoped many more times. such a fast getting better and stay better would be a miracle. it is a pitty.
Hi Konijn
Here is some more info on FMT trials. The Quadram Institute, UK , have very experienced scientists studying the microbiota and they have this information about a 160 person trial involving FMT. The link below has the information but I believe it will be 80 actual FMT transplants and 80 participants who receive a placebo. The trial was due to get underway at the end of 2022 after being held up by the pandemic. I think each person not receiving placebo will receive 10 different donations. No recent updates available.
https://quadram.ac.uk/restore-me-trial-event/
Also please note there is a blog on ‘Invest in ME Research ‘ by Rik Haagmans who is involved in the study and he says the RESTORE-ME study and COMEBACK study (Norway) have joined forces.
Sorry I was unable to share the link but it is titled ‘ME/CFS in the UK and Norway: comparing RESTORE-ME and the COMEBACK study.
Hope you find this helpful.
thank you RT!!! i can not answer on your message. hope you find it. hope we finally get some help!!!
Hi again Konijn
Another reply to your reply. Yes let’s hope that all this interest in the microbiota gives results and treatment that we really need. All the best to you.
RT
yes, RT. we really need some treatment! even if it helps only partial… Ther are so many really at the end of their rope… Decades of illnes and no treatment.
thanks! i did not know that. it is all so complex our desease. And no 2 persons are excactly the same.. I also wonder if a cause could be found in the studys where the microbiome changes before and after “excercise. Or something totally different in the boddy. the persons where you read about FMT, that they did not have long lasting effects, did they have for excample 15 FMT’s? i wonder if there is a difference. also with gender FMT.
Hi Konijn!
Remember you asked me where to get 5-HTP and I spelled it out. Were you able to get some? Did it work?
What else have you tried so far? Like, what do you take every day?
While we wait for potential treatment studies for dysbiosis, we can use prebiotics such as oat bran, inulin, resistant starch, fruits and vegetables that supposedly encourage butyrate producing bacteria. This is in no way suggesting that dietary changes cure MECFS but gut friendly diet may help some of us.
A Personal Slant On Chronic Fatigue Syndrome And The Gut February 13, 2023
I have been experiencing Chronic Fatigue Syndrome for approximately ten years. For the past two years I have been experiencing debilitating recurring cyclic bouts with the consistent symptoms of:
• Swelling and sensitivity in the area of the upper cervical lymph nodes, greater on the right than the left.
• Extreme fatigue
• Muscle and joint pain
• Compromised balance
These symptoms typically last for three to five days and are then followed by a respite of five to seven days.
In an attempt to diagnose my condition the physicians that I consult have ordered numerous medical tests. Two of the tests required abdominal scans both of which were less than optimal due to abdominal gas.
Due to the excessive gas which was evident in the abdominal scans, and the flatulence I experience, ten days ago I decided to try the probiotic Schiff Digestive Advantage containing:
• Bacillus coagulans (1 billion CFUs),
• Protease (30,000 HUT),
• Amaylase (450 DU),
• Lipase 30FIP).
Within a couple of days of starting the probiotic I experienced a significant reduction in all of the symptoms. The change was so rapid and significant that my initial reaction was that it might be a placebo effect, except for the near elimination of the lymph node enlargement.
It was time to start reading, and what a surprise I got.
The NIH ME/CFS Gut Studies Pt. I: A Biomarker for Chronic Fatigue Syndrome (ME/CFS)?
https://www.healthrising.org/blog/2023/02/10/gut-biomarker-chronic-fatigue-syndrome-pt-1/
Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS.
https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(23)00029-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS193131282300029X%3Fshowall%3Dtrue
Bacillus coagulans and butyrate.
https://www.google.com/search?q=Bacillus+coagulans+and+butyrate&rlz=1C1VSNE_enUS652US652&oq=Bacillus+coagulans+and+butyrate&aqs=chrome..69i57.29289j0j15&sourceid=chrome&ie=UTF-8
Good story – most of the problem starts with unwanted pathogens in the gut. Clear the infection and healing should start.
Do you have any info on the pathogens and how to clear them?
Pathogens come in a variety of classifications such as bacterial, parasitic, protozoa, yeast/fungal and viral. I would suspect in your case (guessing) that it likely not parasitic or protozoa since bacillus cannot attack these classes. Most likely is some unwanted bacterial species. I would base some of this assumption on that bacillus coagulans seems to address this. Many probiotic species have antibiotic properties, though their killing power can be specific. In general terms, it is better to use a shotgun approach by introducing a number of probiotics into the body, but I likely would not do that with your situation since you are getting results.
The other ingredients in that formula are digestive enzymes which could also have action against unwanted pathogens. I would stay on that product until you solve the issue or hit a wall.
If you hit a wall then send me a message (best on Twitter) and I can give you more information. Note that I have no commercial interests in helping. Here is my Twitter Patrick231aa
@Patrick33264855
I did make some educational videos about the article Cort lists, let me know if you would like to view. I would like to add that I have many many hours in gut studies and in particular on pathogenic organisms which includes their behaviors, environment and treatment strategies. I had a forum on just protozoal parasites.
Patrick, I think you have called it! I hit a wall. I tried to message you on Twitter and it will not allow me to. My email is Lawson.RD@gmail.com
my Twitter is Robert Lawson@Berto5w4. I never use Twitter and I am unfamiliar with using it. Can you send me an email?
Does starting Bacillus coagulans helped fatigue symptoms of CFS?
Or only helped gastro symptoms?
There is currently a clinical trial evaluating butyrate in Gulf War Syndrome. Preclinical trial results have shown good results.
https://clinicaltrials.gov/ct2/show/NCT05367245
” Also, short-chain fatty acids such as butyrate restored a healthy microbiome and improved gut microbial metabolism apart from attenuating GWI symptom persistence in preclinical studies. Butyrate, a nutraceutical endogenously produced in the host gut following bacterial fermentation, has shown promise in gastrointestinal disturbances such as irritable bowel syndrome (IBS) and Inflammatory bowel disease (IBD).”
One important piece is missing which is that the beneficial bacteria are destroyed due to high ROS (Reactive Oxygen Species). ROS is a byproduct of immune activation. Likely due to pathogenic organisms in the GI tract.
I’ve been exploring the Phair / Armstrong / Davis itaconate shunt hypothesis recently, and they have a remarkably complete story about what might happen to energy, PEM, brain fog, and inflammation if the shunt gets stuck open (in which all the available acetyl CoA gets sequestered before it can be used to make energy). The only thing they’re missing is an explanation of how the stuck-ness that might happen.
Based on this and your Part I article (which mentions severe disruption to the acetyl-CoA based production of butyrate)…..what if the thing that turns off that shunt is butyrate?
According to the leaky gut model, we lose our butyrate producing bacteria, which disrupts endothelial integrity (per other research, not just gut endothelium – the whole vasculature). If the absence of butyrate is the reason cells can’t switch off the itaconate shunt, maybe ammonia and lactic acid might build up in the gut and kill off any butyrate producers, creating a vicious cycle? In that case the conditions would persist regardless of whether the cycle started with an infection, a gut catastrophe, or even some other massive stressor.
Butyrate is known to improve sleep, protect endothelium and downregulate TNFa in other research. This could provide a very detailed but still somewhat tidy unified model of disease, if I’m not just having an apophenic episode.
I had to look up “apophenic” :). Nice word!
“the tendency to perceive meaningful connections between unrelated things.”
I hope you’re on track…
Unfortunately have had too many physical challenges and developed POTS, which I’m still trying to get a handle on 🙁 but hey, two steps forward, one step back….trying a butyrogenic diet to see if that helps, on the speculation that the ME/CFS (endothelial damage, ejection mistiming, or whatever) is the reason for the POTS.
Fingers crossed!
Here’s the Part II youtube video on the immunometabolic piece in case you’re interested
https://www.youtube.com/watch?v=PCnkkLlyVMk
I have managed to raise my butyrate producing bacteria up to 49% according to a recent Biomesight stool test which puts me above average and in the healthy category.
My diversity is up to 92% again supposedly making me healthy but although these results look really good they have made zero difference to my overall health. I still pick up many viruses but do get over most of them very quickly with the help of Andrographis but there is no improvement in my ability to exercise which is quite limited.
I should say I have been working on my gut sueinf the past 2 years especially on improving the butyrate producing bacteria which were low last year. I have managed to improve them by increasing my intake of vegetables and low carb fruit but the main thing I have done is to increase my daily intake of various non soluble fibres like FOS, GOS and many of the others recommended. I have been using a Biocare product which contains mixed fibres and add extra plain FOS. I mix these into a homemade yoghurt with some frozen berries and psyllium husks to bulk it up.
I still have issues with a bit of bloating and intermittent constipation and loose bowels so think I probably have SIBO but the stool test only looks at the colon How I suppose its good to know that my results imply I am a very healthy person which isn’t bad as I will soon be 75!
M,can you tell me the book that David A Bender wrote please.
I’m really grateful to see this piece of research. I’ve had CFS for 15 years and probably a further 2 years, as it was first diagnosed as depression.
Unlike some patients i could not identify a virus that preceeded my condition. The only condition I could remember having was a quite severe incidence of IBS (diagnosed by the GP)< which seemed to resolve itself.
I've recently suffered a worsening of severity of CFS, and one of the new symptoms I've developed is alternating bowel habit between severe constipation, with sttolls like gravel to explosive diarrhoea, thats so severe I sometimes leak faeces.
I've heard theres some resistance to this potential link between the gut and CFS. But it does seem to explain my own experience. Thank you for your efforts.
It does not seem so far fetched, that a condition which impacts so many metabolic functions would also have the potential to impact the brain gut connection.