

Despite its low weight, the brain consumes 15-20% of the blood in the body.
The brain and the blood vessels. So intimately intertwined but so rarely studied together. In retrospect, that’s kind of strange as blood vessels may have more to do with brain functioning than anything else.
By weight, the brain is almost a non-entity – it takes up about 2% of our body weight – but blood flows are another story. The brain receives 15-20% of our blood supply; i.e. it may be the most blood vessel-rich and blood vessel-dependent organ in the body.
Given that we know the spike protein of the coronavirus triggers coagulation, and that several other things (platelet activation, endothelial dysfunction) have gone wrong with the blood vessels, it only seems to make sense to check out the impact blood vessel functioning might have on the brain in long COVID.
Of course, there’s the ME/CFS angle as well. We know that blood flows to the brain are impaired in ME/CFS, and there’s evidence of neuroinflammation, basal ganglia dysfunction, prefrontal cortex problems, etc., but until the coronavirus popped up – except for Ron Davis’s exploration of red blood cell deformability – there was almost no focus on blood factors.
In both illnesses, the brain imaging studies tend to be locked into the brain and only the brain. While blood flows within the brain are often assessed, anything outside of the brain is a no-no. Not so with this PolyBio Research Foundation-funded study.
The Study
That changed with the “Neuroinflammation in post-acute sequelae of COVID-19 (PASC) as assessed by [ 2 11C]PBR28 PET correlates with vascular disease measure” study (it’s currently a preprint) featuring Michael VanElzakker and Marco Loggia.
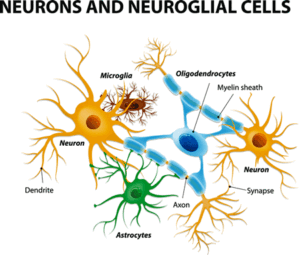
This study assessed the activation of glial cells (astrocytes and microglial cells).
This study asked whether neuroinflammation in the brain and markers of blood vessel dysfunction in the plasma were correlated; i.e. it asked if people with higher levels of blood vessel dysfunction in the blood plasma also experienced more neuroinflammation in their brains.
A positive finding would putatively connect what’s going on in the blood vessels in the body to damage to the brain. We’ve heard of the gut-brain axis. Now we have the blood vessel-brain axis.
The researchers specifically looked at the activity of the glial cells in the brain. The innate immune cells found in the brain, the glial cells are composed of microglial cells and astrocytes.
These are the cells that produce the “sickness behavior” symptoms (flu-like symptoms such as fatigue, pain, etc.) we experience when we encounter a pathogen. In the short term, they play a vital role in clearing pathogens and the damage they’ve caused to our systems. If they remain in a chronically activated state, they may – besides producing a lot of symptoms – produce a lot of dysregulation that shows up in all sorts of neurological symptoms.
The study included 12 long-COVID patients and 43 pre-pandemic healthy controls. The authors used a second-generation ligand [32 11C]PBR28 that can pick up glial cell activation to assess neuroinflammation, and assessed the following factors in the plasma:
- Vascular health (α2- 238 macroglobulin, orosomucoid, CRP [C-reactive protein], fetuin A, fibrinogen, haptoglobin, 239 sL-selectin, PF4 [platelet factor 4], pentraxin-2)
- Cytokines (GM-CSF, IFNγ, IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, 241 IL-8, IL-10, IL-12(p40), IL-12(p70), IL-13, MCP-1, TNFα)
- Angiogenesis (angiopoietin-2, BMP-9, EGF, endoglin, endothelin 243 1, FGF-1, FGF-2, follistatin, G-CSF, HB-EGF, HGF, IL-8, leptin, PLGF, VEGF-A, VEGF 244 C, VEGF-D).
(The plasma is a light-colored fluid that contains what’s left over in the blood after the red blood cells are removed).
They also assessed symptoms using the Brief 252 Pain Inventory (BPI), and the Beck Depression Inventory (BDI). The patients were screened to fulfill a modified myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) International Consensus Criteria (ICC) criteria. Most of the long-COVID cases (10) had not been hospitalized for COVID-19.
Results
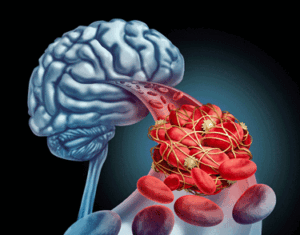
Factors indicating blood vessel dysfunction were associated with neuroinflammation.
“To our knowledge, ours is the first study to provide direct evidence that processes related to neuroinflammation and vascular dysfunction are directly related in PASC (long COVID).” the authors
They found evidence of neuroinflammation (activated glial cells) across a wide swath of the brain in the long-COVID patients. The inflamed areas included the midcingulate cortex, corpus callosum, thalamus, basal ganglia/striatum, subfornical organ, anterior cingulate cortex, medial frontal gyrus, and precentral gyrus.
They really hit the nail on the head with their blood vessel factors. With elevations in 7 of the 9 vascular factors (fibrinogen, α2-macroglobulin, orosomucoid (alpha-1-acid glycoprotein 427 or AGP), fetuin A, sL-selectin (soluble leukocyte 428 selectin, or sCD62L), pentraxin-2 (serum amyloid P component, or 429 SAP), haptoglobin), they found strong evidence of vascular dysfunction.
THE GIST
- Although the brain gets from 15-20% of the blood in the body and may be the most blood vessel-rich organ in the body, brain studies tend to focus on the brain alone. With ample evidence of blood vessel problems showing up in the long COVID, though, that’s changing. A recent PolyBio Research Foundation study assessed both neuroinflammation in the brain and factors associated with blood vessel damage.
- The study found evidence of widespread neuroinflammation in long COVID in several areas of particular interest for ME/CFS. The anterior cingulate cortex, basal ganglia, and thalamus have all shown up prominently in ME/CFS as well. Reduced activation of two of these regions – the ACC and the basal ganglia – has been associated with fatigue and other symptoms in ME/CFS.
- With 7 of the 9 blood vessel damage factors significantly increased in the long COVID patients, blood vessel damage showed up big time. More importantly, the blood vessel factors were positively correlated with the neuroinflammation found; i.e. the worse off the blood vessels appeared, the more neuroinflammation was found. That suggested that blood vessel damage may be contributing to the neuroinflammation in the brain and a wide raft of brain-derived symptoms such as fatigue, chronic pain, flu-like symptoms, and brain fog.
- The authors speculated that remaining reservoirs of the coronavirus were triggering clotting, which was then damaging the blood vessels, and opening up the brain to inflammatory factors via a leaky blood-brain barrier.
These upregulated vascular factors do things like trigger coagulation, glial cell activation, draw immune factors to the blood vessels, and increase the permeability of the blood vessels. Interestingly, the study found increased signs of neuroinflammation in some areas featuring small thin blood vessels such as the basal ganglia, which seem to be more susceptible to damage.
Similarly, parts of the brain called the circumventricular organs (CVOs) lack a complete blood-brain barrier and seem to be “particularly vulnerable to being activated by blood-borne factors.”
These blood-borne factors could be triggering inflammation, putting holes in the blood-brain barrier, activating glial cells (which promote more neuroinflammation and a leakier blood-brain barrier), and wreaking havoc on the blood vessels.
The Brain Regions
Back in 2004, Chaudhuri and Behan proposed that problems in the basal ganglia, thalamus, limbic system, and higher cortical centers were causing ‘central’ or brain-induced fatigue. These areas showed up in spades in this latest study.
Anterior cingulate cortex (ACC)
Of all the brain regions, the anterior cingulate cortex (ACC) and the basal ganglia/striatum stand out with regard to ME/CFS. The ACC appears to be kind of a favorite in ME/CFS. It’s been associated with fatigue in ME/CFS numerous times.
Like all brain regions, the ACC is involved in a number of functions including attention allocation, reward anticipation, decision-making, impulse control – each of which I, at least, have trouble with. The “reward anticipation” is interesting as low reward has been associated with fatigue in the basal ganglia. The ACC also processes a lot of stimuli and is involved with motor control. It appears to kick in when effort is needed to complete a task.
Reduced cerebral blood flow, reduced glutathione levels and perhaps energy production, altered metabolites, altered connectivity associated with increased fatigue, dysfunctional motor planning and reduced acetylcarnitine uptake in the anterior cingulate cortex have all been found in ME/CFS.
Basal ganglia
Found deep in the brain, the basal ganglia have connections to the brainstem, thalamus, and other regions. The dopamine-producing center of the brain and an important facilitator of movement, basal ganglia dysfunction has been associated with fatigue in both ME/CFS and multiple sclerosis.
Andrew Miller – whose CDC-funded study found evidence of significantly reduced activation throughout the basal ganglia – believes basal ganglia dysfunction may be impacting the movement problems in ME/CFS, as well as causing both physical and mental fatigue, increasing the effort needed to do anything, etc.
Some evidence suggests that the basal ganglia may be uniquely vulnerable to inflammation.
Thalamus
Located just above the brainstem, the thalamus’s role in relaying sensory and motor signals from the body to the brain would seem to make it a potentially key factor in multisymptomatic and fatiguing diseases like long COVID and ME/CFS. Another brain region that has cropped up several times in ME/CFS, both the thalamus and basal ganglia have strong connections to the brainstem.
Conclusion
This kind of study which attempts to connect multiple systems together is vitally needed in these complex illnesses. The study was small – but its findings – if validated – loom large. The large differences in neuroinflammation found (no inflammation in any the healthy controls) and the rather dramatic evidence of blood vessel dysfunction (7/9 factors elevated in long COVID) suggested that while the study was small, it was on the right track.
In other words, this was not one of those – if only we’d added another 100 people to the study, this borderline result might become ‘significant’ – type of study. The differences were glaring even with these few patients.
The linkage between blood vessel dysfunction (but not immune activation) and neuroinflammation suggests blood vessel problems could be playing a really major role in these diseases. That’s not exactly new – Klaus Wirth and his colleagues have been postulating that’s true in ME/CFS for quite a while now – but, as the authors noted, it’s the first direct evidence that this is so.
While no one thinks just one treatment is going to do the trick, the study suggests that improving blood vessel functioning might have a significant impact on brain functioning (fatigue, brain fog, pain, movement) and overall health. The cause of the blood vessel problems is unclear but could result when the remaining spike proteins from the coronavirus produce clots that damage the blood vessels.
The results need to be validated in a larger study, but it’s hard to imagine, given this study’s findings, that we won’t see those bigger studies.
Health Rising’s BIG (little) Donation Drive Update
I am still behind in my tallies, but thanks to the many, many people who have supported Health Rising during our end-of-the-year drive.
This is the kind of study – one that links different systems together to provide more complete explanations of these complex illnesses – that I love to emphasize. If that floats your boat as well, please support us in a manner that works for you.






I wonder if microclots could be responsible for my elevated blood pressure after suffering from ME/CFS for two decades?
Maybe it might be worth trying Nattokinase to dissolve the clots?
I remember Dr. Asad Khan said HELP apheresis removed his high seated BP so maybe you are onto something there.
I did 7 HELP apheresis sessions (write-up on this blog) but it did not change my high orthostatic diastolic BP. My seated BP was and remains textbook normal.
I have severe venous insufficiency.
I had four surgeries done to prevent the swelling in my legs. The surgeries did nothing. An Integrative medical doc put me on supplements that “tone the blood vessels from the inside”. Now, I have actual ankles for the first time in decades and my circulation has improved overall.
I can’t believe how drastic the improvement is, especially compared to surgery.
Diosmin+ Horse Chestnut + Butcher’s Broom.
I use a supplement called “Beautiful Legs” (has Diosmin), plus another one that is horse chestnut. There’s also one called Circulegs that can be added to the Diosmin. My instructions were to take them three times a day – just for a week – then once (maybe twice) a day for maintenance.
I wonder the same thing. After 3 decades of low blood pressure and being on fludrocortisone, after my 2nd covid infection I had high blood pressure! Very odd. But gradually over a year that reduced back to a normal level.
I don’t personally recommend nattokinase if not medically supervised. I took natto with aspirin after the 2nd covid infection to help reduce microclots and had a TIA. It was a very scary experience. Room spinning and then when I tried to talk my speech was all slurred. This passed within a few minutes luckily and then I had a terrible headache deep in the brain (not your usual feeling when have a headache or migraine).
Things that are common in both me/cfs and LC, should rule out spike protein in those specifics. As there is no spike protein in pre existing me/cfs.
“Improve blood vessel function”..How?
Actually, the really interesting thing is that if I have it right – it may be possible that there is something like a spike protein in ME/CFS. This is because other coronaviruses that cause the common cold are out there – one of which enters cells through the same receptor that the SARS-CoV-2 does.
I don’t know how different the spike proteins are but if other coronaviruses trigger coagulation something similar could conceivably be happening. One of those coronaviruses can at times put people in the hospital – it’s not necessarily benign.
Oh I forgot about that. Its a really good point Cort.
Taurint próbálta már?Legalább napi 2 – szer 3 gramm a hatékony lehet.
Cort, thank you for sharing this.
I just wanted to quickly share and put on your radar for awareness – the lead report on KSTP’s Channel 5 News Minneapolis – St. Paul Sunday evening (ABC News affiliate) broadcast centered on ME/CFS & LC.
https://kstp.com/kstp-news/top-news/no-treatment-no-cure-patients-await-answers-about-debilitating-disease-related-to-long-covid/
KSTP: “No treatment, no cure: Patients await answers about debilitating disease related to long COVID” (video included)
ME/CFS “has been relegated to the margins of medicine when you don’t have money and funding allocated for it,” he added.
“Earlier this year, the Minnesota Legislature invested an ongoing $3.146 million a year to address the post-COVID illnesses that have affected about 12% of Minnesotans since 2020, she said.”
“Hanlon and Flosdorf said they hope progress in Minnesota spurs more at the federal level as funding for research wanes.”
Just sharing your way,
Dakota
As someone with ME/CFS, I disagree that larger, verification studies should be the next step – as you’ve said, Polybio’s data is significant. In my opinion, next step should be to exhaustively identify a list of medications which might positively address this blood vessels problem, research whether any ME/CFS or long covid doctors have tried them, and then publish the pros and cons of each drug for us to review with our own doctors (including a way to provide feedback on our experience with them to a central location). I’d like to see Polybio repeat their tests with ME/CFS patients and I’d like to see an organized trial of medications but as the years tick by and I continue to have my life upended by this illness I am less willing to wait.
It was good to see those strong numbers in that small cohort – which met the ICC criteria. I wonder if the clot busting and platelet activation meds are being embraced by ME/CFS doctors?
Interesting-In 1994 I had a test which revealed a blood flow problem in the right side of my brain. I also had an EEG and was told my theta brain waves were higher than normal (I think it was an EEG). High cytokines too. Was diagnosed with fibromyalgia.
Excuse me I meant high C reactive protein..not sure about cytokines.
At this rate, I doubt any help is “just around the corner”
” larger studies needed” is all we keep hearing.
Don’t these researchers realize we need help NOW!
What ever happend to bc007 or other proven
Drugs that reverse or(CURE)this horrible disease.
The drugs that reverse or (cure) this, don’t get any larger studies, but instead, almost intentionally, get swept under some kind of hidden carpet.
Yet, if one goes to the youtube brain retraining videos, there are now hundreds of recovery stories.🤷♂️
Look at the billions and billions of dollars raised over decades to find a CURE for cancer yet very few CURES are found, yet many people CURE their own cancers without the use of an oncologist.
There are also many youtube videos of people helping others to CURE cancer.
I suspect, this will wind up being another disease that “we don’t have answers” or “more studies are needed” will reverberate for a very long time.
Personally I’ve been waiting for decades.
I’ve been searching the internet for decades and, in my searches, found most, if not all diseases end up being
” treated ” by big pharma instead of “CURED”
$$$$$$$$$$$$$$$$$$$
I think our big hope is the RECOVER Initiative or other large long COVID efforts identifying possibilities -and yes, it’s amazing how many brain retraining stories are out there. Definitely something to consider.
(…) ”Given that we know the spike protein of the coronavirus triggers coagulation, and that several other things (platelet activation, endothelial dysfunction) have gone wrong with the blood vessels, it only seems to make sense to check out the impact blood vessel functioning might have on the brain in long COVID”(…).
Wow, we also know that mRNA vaccines cause our body cells to produce spike proteins. So if you get 4 vaccinations and contract corona 2 or 3 times, count out your profit….
Yes, Gijs – but studies make it clear that the vaccines to reduce the incidence of long COVID and recent studies suggest they are reducing the incidence of autoimmune diseases. How you react to them – well or poorly – must depend on your physiology.
I don’t agree Cort. It is still to early to conclude that mRNA vaccinations prevent long Covid according to the authorities here in Europe bases on those studies. With regard to claiming prevention of autoimmunity through mRNA vaccination, is very special, because it is known that vaccinations can actually cause autoimmunity. In both cases, causality is difficult to prove.
It remains that the more mRNA vaccinations you take and the more often you get corona, the more often you are exposed to the spike protein that causes the problems you describe. So also more risk of Long Covid. However, there is one big difference: through vaccination, your body produces many, many more spikes than if you become infected with corona naturally.
I doubt that! Please substantiate that with peer-reviewed research.
Coronavirus vaccines are crippled covid virus that has the spike protein, which is
what opens the cell-wall receptor. But lacking the mRNA formula to make full
and viable covid capsids…. your argument does not make sense
It’s definitely caused deaths and injury especially in young men.
We don’t know the effects of these vaccines because there is still no long term data.
”Coronavirus vaccines are crippled covid virus that has the spike protein” This is not correct.
This is how it works. The new mRNA corona vaccines contain synthetic mRNA that carries the code for a certain protein of the coronavirus. That code is converted by your body cells into protein of the coronavirus. This protein is recognized by your immune system as foreign, causing you to form antibodies and other immune cells against the coronavirus. The synthetic mRNA has no influence on your genetic material (DNA). https://www.gezondheidenwetenschap.be/gezondheid-in-de-media/hoe-werkt-het-mrna-coronavaccin-en-is-het-veilig#article-detail-wrapper
the authoritative journal Nature shows that, in addition to the Sars-Cov-2 spike protein, the mRNA vaccines lead to the production of numerous other proteins against which an immune response can be generated in vaccinated persons. https://www.nature.com/articles/s41586-023-06800-3
Wow. The “vaccines” are possibly the biggest crime in human history in terms of compulsory medical experimentation leading to unjustified mortality but especially morbidity. In the next several decades we’ll see (I won’t, I’ll be dead) a significant rise in nearly every disease, with a significantly earlier onset, basically a dramatic decrease in life expectancy and quality of life.
Thank you Cort. VanElzakkers work is really exciting because so far we only had tenuous evidence for neuroinflammation in postviral conditions like LC and ME/CFS. It also validates some of our own work on the role of glia in ME/CFS (https://www.frontiersin.org/articles/10.3389/fncel.2022.888232/full ).
So here the questions start: what´s the hen and what´s the egg? Is neuroinflammation but a consequence of vascular dysfunction? But then vascular dysfunction could be a consequence of other factors – like oxidative stress, which also was shown to be prominent in ME/CFS… or perhaps of viral reactivation (EBV for example also has a predelection for endothelial cells) … or other influences, there are quite a few candidates (https://www.frontiersin.org/files/Articles/888232/fncel-16-888232-HTML/image_m/fncel-16-888232-g002.jpg )
Maybe we can sort things out if we look again at the brain regions most affected in ME/CFS (for a recent review on imaging findings in ME/CFS see https://pubmed.ncbi.nlm.nih.gov/38016575/ ) – by the way, these regions are not only affected by inflammation but also by hypoactivity (which may be another consequence of glial dysfunction). Here we come across the insular and thalamic regions, parts of the cingulate and prefrontal cortex, the brainstem, the circumventricula organs, etc.
Now I am not a neurophysiologist, but these regions all pop up when you read up on the physiological human stress response (here even the circumventricular organs are mentioned: “other important structures for triggering the stress response are the circumventricular organs” ((https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00127/full ) (more on the human stress response here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864527/ )
So this rather directly leads to the most precious recent findings in ME/CFS research, namely the finding that ME/CFS patients have a very specific way of responding to stress: they do not respond at all – as evidenced by
• a lack of metabolic adaptation in response to exercise (“metabolic flatline”): https://www.mdpi.com/1422-0067/24/4/3685
• a lack of transcriptomic adaptation in response to exercise (“transcriptomic flatline”): https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9916639/pdf/ijms-24-02698.pdf
• a lack of changes in circular RNA expression after exercise: https://www.sciencedirect.com/science/article/abs/pii/S0378111923004092
• a lack of adaptation of T- and NK cells after stress exposure: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9916639/pdf/ijms-24-02698.pdf
• a lack of changes in microRNA levels in peripheral blood mononuclear cells after exercise: https://pubmed.ncbi.nlm.nih.gov/32291908/
• a lacking acute phase response to luminal gut antigens translocated into the bloodstream during exercise: https://www.sciencedirect.com/science/article/pii/S2666354623000418?via%3Dihub
• no changes in the total concentration of extracellular vesicles (EVs) post-exercise (which is clearly seen in healthy controls) as well as a delayed increase in abundance of several EV proteins in response to exercise: https://www.biorxiv.org/content/10.1101/2023.08.28.555033v1.full.pdf
• la ack of adjustment of cerebral blood flow in response to orthostatic stress: https://www.sciencedirect.com/science/article/pii/S2467981X20300044
• a lack of cardiovascular and respiratory adjustment in response to exercise (inability to raise heart rate and respiratory rate to the levels demanded by exercise): https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8923458/ and https://journal.chestnet.org/article/S0012-3692(23)00502-0/fulltext
• (Interestingly, a reduced capacity to respond to stress has recently also been found in monocytes and lymphocytes in patients with PASC: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(23)00294-3/fulltext )
So this makes you wonder how van Elzakker´s findings may tie into these findings of a dysfunctional stress response in ME/CFS. One plausible explanation could go like this: because ME/CFS patients do not adapt to stress like they should their whole system will be vulnerable to all the bad things that stress brings along: oxidative stress, nitrosative stress, inflammatory activation, immune dysfunction, endothelial dysfunction, viral reactivation, dysfunctional coagulation and the whole gammut and catastrophies that all sustain the clinical picture of PEM and thus ME/CFS.
And these consequences of a dysfunctional stress response in turn may also sustain neuroinflammation – and thus a dysfunctional stress response. A real vicious cycle.
First – thanks for all the links – I will use them in future blogs 🙂
Really neat to be able to link the problematic brain regions with the stress response. I had no idea.
“Here we come across the insular and thalamic regions, parts of the cingulate and prefrontal cortex, the brainstem, the circumventricula organs, etc.
Now I am not a neurophysiologist, but these regions all pop up when you read up on the physiological human stress response”
Thanks!
Very interesting! Now repeat for ME/CFS and for good measure, for ADHD, too (because symptom overlap).
would having a diagnosis of protein c resistance and factor 5 leidon blood predispose one to fibromyalgia chronic fatigue as in long covid
Interesting, with so many possibilities. I could provide at least a dozen symptoms that I experience as being potential effects of abnormal vascular (or micro-vascular) function – just in the body. If such problems are also in the brain (that controls all systems and functions) as well – as this research seems to indicate – the list of potential symptoms could be extensive (and very explanatory).
I reiterate Bailey’s question; “Improve blood vessel function”… How?
TY again for the great reporting Cort!
Dave W reiterated the question of how to improve blood vessel function.
I have already found some information on that. But I would first like to point out something very important:
that you may need to consider not only vessels, but also all of the organs which are made of Smooth Muscle. Skeletal is striated and normally at rest. Smooth is more smooth-looking as well as-moving.
So when I looked back at The Pandemics since 1918 Spanish Flu, which is mutated but still with us each year, I found that the affected receptors for virus entry were at first Sialic Acid a,2 receptors, in common with avian species, later both that one and the a,6 receptor, and things like swine flu are more attacking the a,6.
Then comes coronaviruses and at least the SARS ones attack ACE2 receptor.
BUT HERE IS THE KICKER:
All three are found on smooth muscle cell membranes, and that’s what makes the long-haul syndromes Systemic. The intestine, esophagus, iris, sphincters, microvessels in the kidneys ALL are smooth muscle, not just the vessels.
Yes, the distribtions of the a,2 vs a,6 receptors are quite different. I have a diagram. But all 3 receptors are attacked in some part of the intestine. Sometimes epithelial cells, other times endothelial cells.
Just to broadly compare flus vs corona, some attack mostly in the nose, some in the bronchi, some in the alveoli.
CHILDREN HAVE MORE ACE2 receptors in their nostrils than adults.
TREATMENT: to limit damage and to repair damage from any endothelial vessel attack, which can lead to death of many elastic cells and remodelling with collagen cells (stiff) — consider these nutrients—-
Citrus Bioflavonoids have been proven in NIH meta-analysis to protect the endothelial cells during attack, and somewhat heal them after attack. This means fresh-squeeze lemon, lime, orange, grapefruit, and ESPECIALLY tangerine. The bioflavonoids have names (incomplete) like Naringen, tangeretin. You also would want Rutin.
This link here is NOT scholarly but it gives the basic considerations.
https://wearefeel.com/blogs/learn/what-is-citrus-bioflavonoids#:~:text=This%20nutrient%20has%20been%20linked,vessels%2C%20which%20is%20called%20vasculitis
.
This next link is
WARNING WARNING WARNING
“”Do not take bioflavonoids without medical advice if you are using warfarin (Coumadin, Jantoven). Other drugs may affect bioflavonoids, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all other medicines you use.””
https://www.cigna.com/knowledge-center/hw/medications/bioflavonoids-d00417a1#:~:text=Do%20not%20take%20bioflavonoids%20without,all%20other%20medicines%20you%20use.
Here is the Scientic Scholarly meta-analysis at NIH:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6431442/
Beyond that
Vitamin E helps to make the vessels more supple,
BUT
WARNING: talk to your doc first and you have to start low dose or you can trigger rapid lowering of blood pressure !!
Ive never heard anything bad about EPA Omega 3 oil from salmon and it does not only heal, it heals because it is a powerful anti-inflammatory. As is Vitamin E.
Lastly, when massive vessel damage occurs, and that stiffer remodelling happens, guess what your endothelials do?
Deposit calcium, to which cholesterol sticks, and then plaque.
You can pipe-clean your vessels if you start grinding golden flax seed into a powder, mix it boiled water, keep stirring so that it does not clump.
Because it’s sticky !!!! And it floats past your cholesterol and removes it in micro amounts kind if like– let’s say– French polishing an antique.
Zinc is known to actually strip the calcium from your vessels. I did not ever know that.
Important:
Vitamin K2 has been used to help covid patients recover better.
Why?
Because it activates calcitonin, which TONES the bone/blood balance of calcium. And in bone, where there’s calcium, there’s magnesium.
You definitely want to discuss all if these treatments first with your doctor.
I had long covid problems with only 2 parts of me: I developed Overactive Bladder Disease on day 6 of my second infection, and along with it my rectum and anal sphincter (smooth muscle) did not have total sensation. I couldnt tell if I were finished or not.
Since starting with Fresh Citrus juice (bioflavonoids), my problems are 95% gone.
And my doctor could never have led me that direction. It is only because I knew that ACE2 gets attacked in ALL smooth muscle. Not just vessels. Who knows what else I fixed !
Chris
Great advice.
But….I have zero calcium in my arteries , at least according to scans a few years ago but I’ve been ill for decades. I definitely believe the vessels are a problem and your ideas will help for sure
TY for the informed and detailed response Christopher!
Too many putative connections are going on in this industry. All they found was a correlation, yet they jump to “ours is the first direct evidence…”(B). And that sentence directly follows the sentence “This is indirect evidence…” (A). Any idea how they got to B from A, Cort? It’s the paragraph starting at line# 546. I looked around just in case I misread or missed something, but I this paragraph just doesn’t make sense. Correlation is not even an indirect evidence in my book, btw.
One possible confounding variable in this case: whatever caused the vascular damage could also have caused the neuroimmune cells to become hypersensitive in same proportion. Therefore, the more vascular damage you have, the more neuroinflammation.
Cort, VACCINES are causing autoimmune issues just like COVID! G.B., MS,and myself small fiber neuropathy!! Just as bad, also causing paralysis!!
Hi Cort,
Hi every person suffering from what I prefer to call SEID. Its the newer name for me/cfs.I think it is most accurate.
Systemic Exertion Intolerance D(isease? (-isorder?)
That said, people disparage the nomenclatures :
Myalgic Encephalomyeltis
and
Fibromyalgia which both exhibit fatigue and non-restorative sleep. Widespread pain.
ME: A brain inflammation that leads to muscle problems as well as musclle pain.
FIBRO: As Cort has analysed, the basal root ganglion at each vertebra is giving redundant pain information. As I learned from a PhD neurologist lecture, when the pain trigger goes from acute to chronic, then at the basal ganglion receptors multiply. Because the pain source does not seem to be dissipating., After that, even if the paim source subsides, the vertebral ganglion for that body region remains on high alert and normal muscle and skin stimuli can come across amplified up to the Reticula Formation at the base of the skull.
Unfortunately, that RF is in charge of brain vigilance: ie: was that somethimg I need to deal with? or can I sleep.
I consider that this prevents restorative (Delta phase) sleep in at least Fibro, but likely in all of female-favouritive autoimmmunities
What is in common, though?
1.
What is in common in ME and FIBRO?
overreaction
CNS inflamation
poor sleep that should, but doesnt reset nerve receptors (nociceptors, baroreceptors etc ) to baseline. There just enough enough deep phase 4 DELTA sleep to quiet the body and allow the Hypothalamus to restore everything, like the grocery store night crew.
Sorry !! :
there just *isn’t enough quality time sleep (restorative phase 4 Delta) to reset the nerves.
If you didnt already know, this is an easy example to grasp:
I walk into the office. Someone’s cologne is way too strong for me. It hurts my nose and maybe I get a headache.
But the receptors (nociceptors— receiving noxious input) dont want to kill us, so they tone down the strength of our REACTION to that cologne. The smell seems less strong.. we acclimatize. But we also get a warning headache.
CRUX: If i sleep well, 8 hours, relatively uneventful, then I can chill, and chill more, and chillax ! into Delta phase 4 restorative sleep where melatonin tells the body to
shut tf up. It’s crucial.
But if something prevents this lack of vigilance by the Reticula Formation at the brain base, then descending into Delta is constantly interrupted.
Why? you may ask
Let me tell you all why:
Chronic inflammation.
It may be brain inflammaton for the most part. We dont know.
But the brain is always “lookin out for
number
one.
Lets say, yes, the brain has inflammation.
Really? How is that even possible?
I’ll tell you:
exact same breakdown of the BBB as happens in leaky gut. Same exact type of TJ )tight junction) cells that are designed to quarantine.
I believe, in all flus, MERS, Gulf War, SARS, and covid, the gut has become leaky
AND
the exact same factors present
affect the exact same TJ cell health in the BBB.
Finally, the Sepsis Association of America declared long covid as a form of Sepsis in 2020 or 2021ish.
What’s that? Septic shock is basically an adverse reactiom of our immune system to extreme attack, often in a hospital bed, im which there is SO MUCH protective CYTOKINE or CHEMOKINE released by attacked endothelial blood vessel cells, that the Hypothalamus says “Ok! Enough! ” otherwise we would de of immne system self-poisoning.
That happens with a fever. But when it outstays its reasonable welcome (because the virus was not eradicated) the HYPOTHALAMUS knows that foreverfever will kill us.
Fever reduces energy metabolism.
If we go into Septic Shock, and stay there (cfs, fibro, long covid, other autoimmune)
what happens?
Dr Naviaux has explained the cellular defense response (CDR) It is unbelievable the biochemstry that he knows. I couldnt finish reading his paper.
Dr David Lam Coaching also discusses neuro-endo-metabolic (cell) defense response.
The Hypothalamus says: ok NO MORE FEVER, you will fry me )your brain) Fever takes much fuel.
During Fever there are about 5 cyto/chemo- kines at work.
In Septic shock, we go into HYPOTHERMIA. it allows us to subsist… since 3/5 cyto/chemo kines stop being produced,
But it makes us cold all day. Amnd the 2 remaining Cyto/Chemo kines perpetuate chronic inflammation.
Voila !
And the ENERGY problem with BOTH fever and hypothermia…
is…
wait for it…..
Drastically reduced mitichondrial energy production because it aint the optimal 98.6 F or 37 C.
What option does the cell then have? Anaerobic metabolism usimg glucose , at a fraction (1/13th) of ATP production compared with oxygen-based metabolism.
Byproduct? Excessive lactic acid buildup with slow clearance= post exertional malaise (PEM)
Chris
I think you’re correct Chris but I think the one added thing to remember is that CFS is likely phenotypical. You have to have these faults in the body that allow leaky gut and dodgy blood bain barriers. This runs in families. Why?
Dodgy connective tissue. We’re just more vulnerable . That’s what allows the cascade of events you describe to happen.
There is a paper that found hyperactive neutrophil in FMS. When they transplanted the hyperactive neutrophil to mice, they exhibited FMS symptoms. If replicated, that is about as direct an evidence as it gets. In MECFS, there are enough reports of hyperactive microglia. And they are now finding both hyperactive neutrophil and microglia in long-COVID. Neutrophil and microglia are the same type of innate phagocytes. Maybe both neutrophil and microglia become hyperactive in some people after certain infection/stress, and that could explain 60% comorbidity of FMS and MECFS.
Nicotine can help!
https://link.springer.com/article/10.1007/s10863-019-09800-z
I dont think this has been posted here before.
https://www.sciencedirect.com/science/article/pii/S1567724923000351
But we dont possibly need to wait years for Robert Naviaux to come up with his miracle drug Suramin as Nicotine can do the same job! https://www.sciencedirect.com/science/article/abs/pii/S0924977X2030938X?fbclid=IwAR23cffZUZ8EwzQjJ900RqdxreDX9bAfafbsYL30JoO7Q3fdpm3ao1Si7Dc
Wallace,
you nailed it.
I started studying viruses because of covid. I started in March. 2020.
What resulted? People with pre-exisiting CvD (cardiovascular disease) which is
inflammation of the endothelial vessel cells, fared the WORST or died when hospitalized
with covid.
It is important to learn that Obesity or metabolic syndrome are the RESULT of CVD and of liver dysfunction. When the liver is overtaxed with toxin filtering, the first function to be cancelled is the bile-directed metabolism of lipids. Why? Because the safest way to deal with toxins that are too volatile is to store them in fat…Sometimes the toxin is Glucose. Glucose should only be blood sugar if needed at the magic level of 4gm. The brain needs there to be always 4gm to draw from, and will always favour itself and hog 40% of glucose. HOWEVER: the brain’s preferred fuel is Ketones. They come from lipid breakdown by the liver. If it’s still working.
So what was found in covid was that the top 3 co-morbidities leading to poor outcomes in hospital, or even death, were:
1. CVD
2. CVD evident as diabetes
3. CVD evident as obesity
but they are ALL vascular inflammation that lead to poor response to the heart, then the heart does all the work, and that’s when the C-disease comes in.
The GIST: At the start, the doctors felt covid was a pulmonary problem. But leading Doctors such as Dr. Roger Seheult, who has 4 separate board certifcations, and who prefers pulmonology, noticed that THE LUNG MUSCLES WERE WORKING JUST FINE. It was about lack of oxygen absorption in the epithelial but inner lining tissues of the alveoli. At that point… as a LUNG SPECIALIST, he concluded that covid doesn’t kill by defeating the lungs. Covid kills by being a systemic attack on ALL SMOOTH MUSCLE, system-wide. Which, in the majority is vasculature, but gets quite critical in microvessels in organs, notably the kidneys.
Roger noted that smokers were expected to die, because they punish their lungs constantly. But among smokers who has serious covid perhaps meriting hospitalization, or who WERE hospitalized, SMOKERS has the LEAST destructive outcomes from covid.
Here is why, Wallace: Nicotinic type Acetylcholine receptors are used for three main functions:
1. Muscle flex and CONTINUED EXERTION (long-term potentiation)
2. Memory and cognitive function (also long-term potentiation to keep facts in memory and to process them)
3. Analgesia (pain relief system in the brain to the body)
An Acetylcholine receptor, whether Nicotinic type (they noticed it likes smoking), or Muscarinic (they noticed that one likes certain mushrooms)
also welcomes similar substances that are not ACh:
they like: nicotine, choline by itself, and I think B12 or B6 (must get back to you on the 3rd one.)
quite possibly the nicotine has molecular mimicry going on.
But read this from the Wiley Online Library:
“It is now believed that there exists a cholinergic ANTI-INFLAMMATORY PATHWAY through nicotinic acetylcholine receptors. Thus, activation of these receptors (particularly a7 nAChR) [hich is nicotinic Acetylcholine *Receptor] , can suppress production of pro-inflammatory cytokines, as these receptors are abundantly expressed in a variety of immune cells including B cells, T cells, and macrophages…..[and] to inhibit tumour necrosis factor TNF-a, interleukin-1 (IL-1) and IL=6
WITHOUT AFFECTING the anti-inflammatory cytokines such as IL-10.” I can provide bibliographic info if needed.
And they go on to say that in situations such as sepsis [too many cytokines in covid] ,
and in ischemia-reperfusion (ask Cort Johnson about that one, where you lose blood access, lose oxygen, then the sudden return does damage)
“in animal models…they showed improvement by stimulation of the VAGUS NERVE (see Cort’s blogs) “which is believed to be mediated via activation of a7 mAChRs on Macrophages.
Finally, what Dr. Roger Seheult told us on Youtube (Covid -19 update # 63) is that if a person who already has obesity, diabetes, or full-blown Cardiovascular disease, covid has a HUGE chance of killing them. Why? Because in those vascular conditions, 2 out of the 3 antioxidants in cell metabolism may be impaired. Then, when covid blocks your ACE2 receptors, which are made to accept angioTENSin 2 enzyme (converter) to lower blood pressure on the fly, every heartbeat ! ….
….the enzyme can’t dock. Normally, if it did, the a7 nAChR would receive a cascade signal from that, and would tell the inner-vessel (endothelial) cells to produce the 3rd possible intra-cell antioxidant to reduce all those cytokines/chemokines. And covid blocks that cascade.
And that is, as Dr. Seheult postulated, with multiple paper citations,
“maybe…. just maybe…. why people are dying from covid”
I have been studying covid for 3 years, plus physiology, biochemistry, functional medicine, anatomy, the HPA axis and neurology . But I am simply an apt pupil, a scientist at heart, who resents mysteries. I have no medical certification.
Does low blood flow to the brain cause neuroinflammation?
From my research…. NO. Neuroinflammation occurs when insults enter the brain.
They may be foreign food particles, viruses, bacteria, and even your own BODY
immune cells which have NO BUSINESS being in your brain.
The most likely cause of neuroinflammation is microglial activation against
foreign matter. And it has been established that some pathogens add on
some info to your DNA. This is because they have partial mRNA, which
is messenger RNA structure, and this structure is enough to allow them access
into the cell nucleus where your DNA are highly protected.
One of the most likely interpretations, then, is that there is foreign
information now stuck to your DNA and sometimes the immune system
notices, and sometimes it is quiescent.
If you lack oxygen in your brain…. this is bad news. your brain function will suffer,
and there may be some loss, as in a stroke situation. At the same time,
people who have sleep apnea and then get a cpap machine, make a full
recovery when oxygen supply is restored to normal.
Brain inflammation is going to be an adverse or attacking reaction,
including heating up, to destroy foreign matter.
I don’t see how a lack of oxygen or glucose or ketones would
cause inflammation. That said, maybe I know nothing,
and maybe I said just enough for you to research the right
subject.
What I CAN be sure of is this: if your vessels have been damaged
and Ischemia results (reduced blood flow to any area),
then you will have loss of function and maybe of memory.
HOWEVER:
I had a long-covid overactive bladder disease, and , embarrassingly,
“dumb rectum disease” simultaneously in which needing to pee
went from “Oh maybe I need to…” to “OMG it’s an emergency”
with pain, and not even full , shall we say “Closure’ on the subject.
But I found a solution: it is Citrus Bioflavonoids, which are most abundant
in freshly squeezed juices of Lemon, Grapefruit, Lime, Orange…
and apparently tangerenin or tangerines, are the bomb.
I have managed to bring these functions back to normal,
without such urgency, without such pain. I interpret this as MOSTLY thanks to Lemon juice,
but I think grapefruit is #2….and where the heck can i buy tangerine juice?.
I have an NIH (U.S. National Institute of Health) Meta-Analysis article on how citrus bioflavonoids can
a) prevent or mitigate vessel and capillary damage during disease attack,
and
b) heal the damage.
I am grapeful for grapefruit !!
The recurring theme is that big studies are needed to solve ME/CFS and now Long Covid.
Well, how is this for a really big study.
“Increased risk of chronic fatigue syndrome following infection: a 17-year population-based cohort study”
https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-023-04636-z#:~:text=Conclusion,increased%20risk%20of%20developing%20CFS.
From 2000 to 2017, researchers in Taiwan included a total of 395,811 cases aged 20 years or older newly diagnosed with infection. The cases were matched 1:1 with controls using a propensity score and were followed up until diagnoses of CFS were made.
The study found: Our population-based retrospective cohort study found that infection with common pathogens, including bacteria, viruses, is associated with an increased risk of developing CFS.
Triggering events—such as pathogen exposure, metal exposure, and environmental factors—initiate immune responses and generate oxidative stress that harms mitochondria [7,8,9,10]. The accumulation of stress through these triggering events elicits autoimmunity in genetically predisposed populations and eventually leads to clinical manifestation of diseases.
Additionally, inflammation dysregulates the HPA axis [12], resulting in a vicious cycle in which hypocortisolism worsens control over the production of proinflammatory cytokines [8, 9]. Furthermore, circulating cytokines increase blood–brain barrier permeability, activate glial cells, and sensitize neurons to non-noxious stimuli [13]. These manifestations of neuroinflammation may explain the neurological symptoms of CFS, such as fatigue and pain.
Various pathogens; including viruses and bacteria such as Borrelia burgdorferi, Coxiella burnetii, Chlamydia pneumonia, Mycoplasma, Mycobacterium tuberculosis, H. pylori, Salmonella, Campylobacter, and Escherichia coli have demonstrated the capacity to “trigger” CFS [14,15,16,17,18,19,20,21]. In fact, some patients have reported having a virus-like illness before the onset of CFS [22]. The association between CFS and viruses including Epstein–Barr virus (EBV), cytomegalovirus (CMV), human herpesviruses 6–8, human parvovirus B19, enteroviruses, lentivirus, Ross River virus, and varicella–zoster virus (VZV) has been demonstrated to various degrees [23, 24].
I won’t quote further from this paper, but it deserves a careful read since it is the largest study of its kind that I am aware of.
This sounds like a very interesting study Betty – that has connected some dots. Thank-you for sharing!
Thanks again for a great article.
What are some interventions/meds/supps one can do /take to specifically target blood vessel functioning?
You write: “We know that blood flows to the brain are impaired in ME/CFS, and there’s evidence of neuroinflammation, basal ganglia dysfunction, prefrontal cortex problems, etc., but until the coronavirus popped up – except for Ron Davis’s exploration of red blood cell deformability – there was almost no focus on blood factors.”
There has been work, but it has been ignored. Prof Durval Costa did several studies with Spetscans – I remember one from the mid 1990s. But there being no interest in the results he moved into other studies.
Right – that’s what I said – there’s been almost no focus on blood factors. There were the Berg studies in the early 2000s and Dr. Costa – who I did not know about – and that was it. Things have definitely changed :). Thanks for the tip about Costa.
There are only 2 main factors in low pressure:
the electrolytes sodium and water.
I order for the sodium level to be balanced, it must be in ratio with water.
My reading of CFS symptoms plus basic physiology indicates that if:
the adrenal glands are knocking themselves out to overproduce Cortisol for inflammation management, then likely production of the salt-retention hormone Aldosterone will be neglected.
In the kidneys, if sodium is recognized as being lacking, then Aldosterone will signal the kidneys to RETAIN sodium.
The type of ACE2 or Sialic acid 2,2 or 2,6 receptors hijacked by
covid, or in the past Flu Pandemics
is not so important as the fact that both types of cell membrane receptors can be globally attacked in either covid, OR flus etc.
They are both present in endothelial vessel cells– and the kidneys are particularly susceptible to the damage since kidneys contain microvessels. That is where osmosis , or glomerular filtration needs to happen 24 hours a day.
The bottom line is this:
1. CFS patients are quite aware that they have cerebral inflammation
2. This, plus all autoimmune activity in the body itself, underlines the need to control or tone down
chronic inflammation. There are Indian root and Chinese mushroom anti-inflammatiry herbs = adaptogens tgat have been consumed for centuries.
If the inflammation can be tamed, then recovery and nourishment of adrenals and kidneys can begin.
If this is not addressed, then negligible sodium retention via Aldosterone from the adrenals will continue. This will allow sodium to be excreted, and that requires water to be excreted in ratio. That will all perpetuate low blood volume, which equals low blood pressure
Finally: a net low volume of water in the blood will have an osmosis ongoing effect of low CELL volume.
Low cell volume is enough to deform the healthy shape of cell membranes.
The membrane nooks and crannies are very specific to match the shape of insulin and even acetylcholine receptors. A deflated cell volume is chronic dehydration. Insulin resistence, then is only natural.
Therefore one needs to lower amd control inflammation, in order to give the adrenals a break from cortisol production, allow Aldosterone production to rebound, restore blood volume and pressure.. amd so on.