Geoff’s Narrations
The GIST
The Blog
The GIST
- Recent studies suggest that a hypermetabolic state that damages the mitochondria results in a hypometabolic state in chronic fatigue syndrome (ME/CFS), long COVID, and fibromyalgia (FM). They also suggest that something in the blood, serum, or plasma is damaging the mitochondria in these diseases.
- We’re not done with the mitochondria, though – far from it! Now we look at a bevy of recent long-COVID mitochondrial studies suggesting that mitochondrial dysfunction affects more than energy production and which illuminate what may have gone wrong in the mitochondria.
- Muscle biopsies of 120 long-COVID patients who had ended up in the ICU found that a year later their muscles had higher levels of immune cells involved in tissue repair and reduced activity of the 2nd and fourth mitochondrial complexes. The authors concluded that there was “aberrant repair and altered mitochondrial activity in skeletal muscle.”
- They couldn’t explain how a respiratory illness affected the muscles but a subsequent study did. A hamster model found that the coronavirus suppressed the genes associated with the muscle fibers, protein production, both sides of the mitochondrial energy production process (Krebs cycle and electron transport chain), and fat breakdown.
- As it was doing that, it unleashed a barrage of inflammatory factors (IFN-α, IFN-γ, and TNF-α) which triggered a shift from relying mostly on aerobic energy production to the less effective process of anaerobic energy production (glycolysis).
- The authors concluded that using treatments “that can boost mitochondrial functions, enhance protein synthesis, and inhibit protein degradation” may be useful for treating muscle fatigue in long COVID.
- Next, a muscle study assessing “maximal fatty acid oxidation (MFO)” (i.e. energy produced by the breakdown of fats during exercise) found significantly reduced levels of fatty acid oxidation in long COVID and a “premature shift” from relying on fats to carbohydrates to powering their cells.
- This was important because the body prefers to burn fats during exercise and because fats play key roles in both parts of the mitochondrial energy production process. The finding wasn’t so surprising, though. Problems with carnitine – which transports fatty acids into the mitochondria – have popped up in both long COVID and ME/CFS – suggesting that the fatty acids that power the mitochondria during exercise may not be getting into them.
- A review paper asserted that increased free radical production (reactive oxygen species (ROS)) by the mitochondria both pushes the cell into a state of anaerobic energy production but also pushes the immune system to activate the inflammatory or innate immune response and away from the adaptive immune response that targets pathogens. This benefits the viruses by providing the substrates they need to grow and allows them to escape from the immune system.
- Several researchers, including Avindra Nath, believe that the immune system tries to compensate for the impaired adaptive immune defense by ramping up the innate immune response. Nath believes this shift plays a central role in ME/CFS.
- They proposed that treatments to boost mitochondrial functioning and reduce the production of mitochondrial reactive oxygen species (ROS) (free radicals) will be beneficial.
- Lastly, a review asserted that the predominant view of the mitochondria as the main energy producers of the cell is misguided and incomplete. Harkening back to Naviaux’s characterization of the mitochondria as the primary threat-sensing part of the cell, the authors believe the mitochondria regulate the “physiological processes at the level of the cell, organ and organism”; i.e. the mitochondrial problems affect much more than low energy levels and fatigue.
- A blog on red light/infrared light therapy – which could both boost mitochondrial health and antioxidant defenses – is coming up.
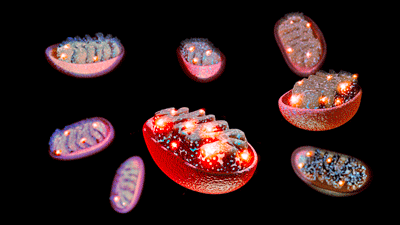
Damage to the mitochondria – the energy centers of the cell – may be affecting more than energy production in ME/CFS and long COVID.
Health Rising recently reported on studies suggesting that a hypermetabolic state that damages the mitochondria results in a hypometabolic state in chronic fatigue syndrome (ME/CFS), long COVID, and fibromyalgia (FM). We also reported on studies suggesting that something in the blood, serum, or plasma is damaging the mitochondria.
We’re not done with the mitochondria yet, though – far from it! Now, we turn to a recent spate of mitochondrial studies in long COVID, ME/CFS, and FM.
First, long COVID. While there’s always been some interest in the mitochondria in long COVID, it’s picked up dramatically in the past year or so and it seems like we’re seeing movement in this issue.
Whacked Skeletal Muscles, Oh My
The first study examined 120 long-COVID patients who had ended up in the ICU – not our typical group. A follow-up indicated that almost all of them suffered from post-viral fatigue and did poorly on a six-minute walk test (@45% of normal).
Muscle biopsies done almost a year later found a higher abundance of M2-like macrophages (which are involved in tissue repair) and satellite cells and lower activity of the 2nd and fourth mitochondrial complexes. The authors concluded that “aberrant repair and altered mitochondrial activity in skeletal muscle associates with long-term impairments in patients surviving an ICU admission for COVID-19.”
I was unable to get this study, but the obvious questions here are: how does a respiratory infection like COVID-19 end up damaging the skeletal muscles, and why are immune factors involved in tissue repair still apparently trying to repair the muscle fibers almost a year after the infection?
 Putting the Pieces Together?
Putting the Pieces Together?
A recent US study, “Respiratory SARS-CoV-2 Infection Causes Skeletal Muscle Atrophy and Long-Lasting Energy Metabolism Suppression“, had an answer for this. This study used a hamster model to suss out what was happening in the skeletal muscles after a coronavirus infection.
The fact that the virus did not invade the muscles but still produced atrophy of the muscle fibers brings up the question of how it managed to do this.
It appears that the virus initiated two processes that allowed it to do this. For one, it suppressed the genes associated with the muscle fibers, the ribosomes (which produce proteins), with mitochondrial metabolism (energy production) as well as genes involved in fatty acid B-oxidation (a key source of energy for the mitochondria), the TCA or citric acid or Krebs cycle and all five of the complexes found in the electron transport chain. The gist is the virus turned off genes involved in energy production.
Note that there are two parts to aerobic energy production. First, the TCA, or Krebs cycle, produces electron carriers (NADH, FADH) and then provides them to the OXPHOS complex (electron transport chain). OXPHOS then produces the end result – ATP. Somehow, the virus downregulated genes associated with both these complexes!
As it was doing that, it unleashed a barrage of inflammatory factors (IFN-α, IFN-γ, and TNF-α) which triggered a shift from relying mostly on aerobic energy production to anaerobic energy production (glycolysis). Since it also downregulated genes associated with muscle fibers, it’s no wonder the hamsters’ muscles were in such poor shape.
The authors concluded – in what looks like the beginning of a nice treatment regimen – that targeting TNF-α during acute SARS-CoV-2 infection to tone down the inflammation that triggers the energy “shift”, and then using drugs “that can boost mitochondrial functions, enhance protein synthesis, and inhibit protein degradation”, may be useful for treating the muscle fatigue associated with long COVID.
- Looking forward to – the Open Medicine Foundation’s deep dive into the muscles, David Systrom’s examination of the muscle biopsies used in his mitochondrial treatment study, and Paul Hwang’s work on WASF3.
- Dig Deeper! Check out WASF3 – NIH Researchers Find New Mitochondrial Abnormality in ME/CFS
The Muscles Pt. II
A muscle study, “Maximal oxidative capacity during exercise is associated with muscle power output in patients with long coronavirus disease 2019 (COVID-19) syndrome. A moderation analysis”, assessed “maximal fatty acid oxidation (MFO)”; i.e. energy produced by the breakdown of fats during exercise.
In fatty acid oxidation, fatty acids are transported into the mitochondria via carnitine where they are broken up and then enter both the TCA cycle (where the electron carriers are created) and the electron transport chain where ATP is finally produced; i.e., fats play key roles in both parts of the mitochondrial energy production process.
Fat breakdown is an important aspect of energy production during exercise because during “moderate exercise” (45-65% of VO2 max) the body mostly uses fats as its primary fuel source. This study found significantly reduced levels of fatty acid oxidation in the long-COVID patients and a “premature shift” from relying on fats to carbohydrates to power our cells.
This wasn’t so surprising. Problems with carnitine – which transports fatty acids into the mitochondria – have popped up in both long COVID and ME/CFS – suggesting that the fatty acids that power the mitochondria during exercise may not be getting into them. Rob Wust, in fact, recently found that muscle atrophy was associated with reduced fatty acid oxidation (energy production) in long COVID.
Importantly, the authors noted that in cases like this, dysfunctional mitochondria consume excess oxygen but produce less ATP (energy), resulting in an increased production of the same reactive oxygen species (ROS or free radicals) featured in the studies above.
- Looking forward to…Maureen Hanson’s work on metabolomics and hers and Rob Wust’s continuing work on lipid and carnitine dysregulation
A Core Breakdown Identified?
Meanwhile, in “SARS-CoV-2 mitochondrial metabolic and epigenomic reprogramming in COVID-19“, the Center for Mitochondrial and Epigenomic Medicine reported that an exhaustive search” revealed that the coronavirus not only “strongly inhibits mitochondrial oxidative phosphorylation (OXPHOS) (aerobic energy production)” but also increases mitochondrial reactive oxygen species (mROS); i.e. free radical production.
This paper, like the review below, proposed that mitochondrial breakdown does more than impact energy production: it also radically alters immune system functioning.
Note that authors believe that increased free radical production (reactive oxygen species (ROS)) pushes the cell into anaerobic energy production. A similar idea was proposed by Stanford researcher Vishnu Shankar, whose ME/CFS T-cell study found that higher ROS levels resulted in increased T-cell proliferation – not necessarily a good thing. Shankar believes that all that T-cell proliferation puts a greater strain on ME/CFS patients’ probably-already-damaged mitochondria – resulting in the production of even more reactive oxygen species (ROS) – and more damage to the mitochondria.
The immune shift engineered by viruses (through the production of “DAMPS”) is an intentional one to distract the immune system and get cells to provide the substrates the viruses need to grow. High levels of reactive oxygen species ROS (free radicals) levels release mitochondrial DNA (mtDNA), which then triggers the activation of the innate, or early, immune side of the immune system.
That’s not a good thing, as the innate immune system is responsible for much of the inflammation produced by the immune system but is not designed to combat pathogens. The part of the immune system that is – the adaptive immune side – is sidelined, resulted in impaired pathogen defenses and increased inflammation.
Several researchers including Avindra Nath believe that the immune system tries to compensate for the impaired adaptive immune defense by ramping up the innate immune response. Nath believes this impaired adaptive immune response is the key driver in ME/CFS.
The authors believe this process triggers epigenetic alterations that suppress aerobic energy production long after the virus has been vanquished. They noted that all the common symptoms of long COVID (and thus of ME/CFS as well) (post-exertional malaise, fatigue, brain fog, dizziness, gastrointestinal symptoms, heart palpitations, hormonal alterations, thirst (blood sugar alterations), chronic cough (inflammation), chest pain, and abnormal movements (cerebellar effects)) have been found in mitochondrial diseases.
Given that, they proposed that the most effective therapies will include treatments to boost mitochondrial functioning and reduce the production of mitochondrial reactive oxygen species (ROS) (free radicals). Shankar found antioxidant treatments like NAC, metformin, and liprostatin-1 reduced the problematic T-cell proliferation he found in culture.
- Looking forward to Shankar’s paper on oxidative stress. continuing work on energy production and the B-cells from the UK, (and a blog on mitochondrial repair).
The Core Breakdown – Take II
Three other review papers over the past year have explicated how a coronavirus infection may be impacting energy metabolism and the mitochondria in long COVID. One asserted that the predominant view of the mitochondria as the main energy producers of the cell is misguided and incomplete.
Harkening back to Naviaux’s characterization of the mitochondria as the primary threat-sensing part of the cell, the authors think of the mitochondria as central cellular processors that integrate signals from inside and outside the cell and then send out signals that regulate “physiological processes at the level of the cell, organ and organism”. In their conception, the leaky electron transport chains in the mitochondria of long-COVID patients even have something to do with their leaky guts; i.e. the mitochondrial problems affect much more than low energy levels and fatigue.
- Looking forward to…more comprehensive reviews of the potential impact of mitochondrial breakdown in these diseases.
Conclusion
Poorly functioning mitochondria may be doing a lot more than simply impacting energy levels: they could be causing an immune shift that impairs our ability to fight off pathogens and pushes our immune systems more toward allergic (and mast cell) responses. Plus, as Naviaux pointed out some time ago, they may have a broad impact on physiological processes across the body. Indeed, Dr. Martin Lerner was fond of saying that increased energy levels took care of virtually all his ME/CFS patients’ symptoms.
We may also be getting closer to understanding why the mitochondria have gone wrong. Problems with fatty oxidation, for instance, may be preventing fatty acids from getting to the mitochondria. Infections may be shifting the mitochondria into a hypermetabolic state which exhausts them. Why that would happen in ME/CFS and long COVID we don’t know, but the production of increased reactive oxygen species (free radicals) could be pushing our cells to rely on anaerobic energy production. Whether that is a secondary phenomenon caused by mitochondrial breakdown, or whether a breakdown in our antioxidant defenses is to blame, or both, is unclear.
Red light / infrared therapy is an intriguing possibility since it could boost both mitochondrial and antioxidant production. While no one expects it to be a fix for these diseases, it could help. A blog is coming up on that.





Thank you Cort for annother hopeful article. My functional medicine Dr prescribed Wobenzyme plus enzymes for MECFS muscle pain with movement, and they do help me. They must be taken 45 minutes before meals. I’m curious to know if we should be trying to move (within our energy envelope) through the muscle pain or if we are doing permanent muscle damage by doing so?
Hi ‘Seattle’
I don’t think that anyone yet has a definitive answer to your question. As you mention, exercising within your energy envelop and pacing yourself may help to avoid damage to your muscles.
I’ve been working to rebuild my muscle strength and endurance over the last two or three years. There’s a protocol that I follow involving careful attention to diet, supplements, and stress management. For me, following it has led to progress. If I over-do it, the consequences involve PEM.
I frequently write and post on Substack regarding mitochondrial involvement in Long COVID. Posts are freely available here https://longcovidjourney2wellness.substack.com/
Kind regards,
Mardi
EXCELLENT information !
Interesting that Shankar mentions metformin, when Systrom is trialling that + LDN. Will be interesting to see how that trial goes
I thought it was Mestinon.
Yes you are right!
Thanks for info on Urolithin A which I’d not heard of, but have now pursued on the web (“Mitopure” is useful re 15 years’ research).
However looking for it in powder form on Amazon & eBay, it all seems to come from China, but very rarely with any kind of quality endorsement for something so expensive: at ~£300 per kilo (the cheapest) for the only kilogram of it I could find, on eBay. Smaller quantities were far more expensive: eg ~£200 for 500g or ~£70 for 100g.
All these were listed as “99% purity”, but the ONLY one with any kind of testing info (quite comprehensive) was the £70/100g bag with a “Certificate of Analysis” from the Shaanxi Zhenge Phar Bio Co. Ltd. None of the others had any name or address of origin.
Do you recommend any particular brand?
PS Sorry I should have addressed the reply above to Ann1
Gabriel:
I posted the link to the one I buy down below. I have been using it and I like it. Mitopure doesn’t have enough Urolithin A and the additions do nothing. It is pure branding, marketing, to say, “Hey, we’re different!”
I’ve never had a problem with anything from China, but I have had neverending problems with things I order from the States. I’m in Canada.
Gabriel:
The brands you see on Amazon, like Mitopure, are made from Urolithin A powder imported from China, then they add “something” and say it’s made in USA. There are no pomegranate orchards in the US. It’s cheaper to get it from the source, and I have found that the producers fall all over themselves to “make it right” if something goes wrong, like shipping failures. YMMV.
Gabriel:
I was wrong! There are some pomegranate orchards in California, but they don’t make Urolithin A from the fruit, they sell it as a whole fruit.
No worries 🙂
Ha ha !!! So I’m not the only one who was trapped in the web of drug names.
ok… weird.
I had clicked the REPLY button for the comment on metformin/mestinon
Cort, you’ve been promising a red light blog for months! 😉 It has helped me a lot, but you can’t just buy a small and weak set of lights for this, and you can’t expect instant results, either.
Ask me, I’ve been doing this for years now.
Also, Urolithin A encourages mitophagy, in which old and damaged mitochondria are destroyed. This has also helped me, but you have to take a lot of this to get the effect.
I will detail all this when you post your blog.
How much urothilin A you take?
There are many brands of Urolithin A to choose from on Amazon. Many of them include extra additives that do nothing. Start with 500mg of Urolithin A with each meal for two weeks, then go up to 1000mg with each meal.
Yes, this gets expensive. I buy 99% pure Urolithin A powder by the kilogram on Ebay. Then I fill a 000 capsule with the powder and take one with each meal. I tried taking two, but it didn’t work any better so I went back to one. I think each 000 capsule will hold 1500mg, but I have not weighed it.
Hi Ann1,
May I ask what symptom/s the Urolithin A helps with?
Matthias,
It helps with body and joint pain, stiffness, and gut dysbiosis.
Thanks. Not mental fatigue?
Not much, if any. LDN is what helps my mental fatigue.
Can you post the ebay link to the product you’re buying?
Nomen,
https://www.ebay.com/itm/205050709386?_skw=Urolithin+A+powder&itmmeta=01JB5CD72747HCS3XWERV8932Q&hash=item2fbdf9858a:g:SMcAAOSwUGNk4zKV&itmprp=enc%3AAQAJAAAA8HoV3kP08IDx%2BKZ9MfhVJKk2JGatsCxgfZH5HKESJO2PpiAGIfTi2viIjpMIls16mWqP2%2BzvC2aast7avoOTtlfQZkq1J4lZKdx86y2CNfuC5GzYiggCDx%2BN0Tj3PEWiJ8wA3TQjmYsHNulCzUvvYIz%2BS8Zrw%2FQDM2M%2FvnCpvNwq1Kl6VxkLJGR8N%2Fdk0OFSAkKAo%2Biw3nWplN0YW78Jo08g0aCir4OgRXkvP528y1YR75S79Myo1FhwXRohqz0vSe7DVq6ZUCinUQySlKKbcGMhrA%2B927tsvf146YoPAD897UpCSHgnGvbyjXlavcp8Ug%3D%3D%7Ctkp%3ABk9SR5rxtKzZZA
Ann1,
EXCELLENT encouragements. 2 of them:
-Red light therapy can impart energy to mitochondria, even when
mitochondria are “down in the dumps” with regard to chemical motivation.
-Mitophagy is perhaps the BEST HOPE of a CFS patient.
Why?
Because Cort is describing to us “mitochondrial damage.”
If we could somehow replace damaged mitos with perfect ones,
then maybe they could go BACK TO NORMAL.
Ok, well, that’s bad. But it is not incurable.
Our Mitochondria have at least 3 (KNOWN) Phenotypes depending
on the chemical *conditions* in our cells.
They have been desribed not only as the energy generators
of our cells,
but also as
the canaries in our coal mines (of our cells.)
Here is an example:
If we have flu or covid or some dastardly bacterium infection,
the HYpothalamus will create a new temperature set point:
It will be FEVER. This is to incapacitate our own ribosomes
and other producers in our cells which need 98.6 F or 97.0
such that: try as they might to reproduce out of control,
our own cell factories do not have enough energy for that.
THIS creates a logistical advantage: It buys TIME
With 3, 4 or 5 days… worst case 14 days… our Immune system
can create antigen-specific ANTIBODIES against the attacker.
AS I SEE IT:
in CFS/long covid, the FEVER pitch has been set,
but never truly lets go.
You may have unexplained and intermittent fevers,
as part of your “flu-like” symptoms.
But let’s get a grip: (but not: “la grippe” !)
Flu symptoms are as follows, for a good reason:
1. Raise body temp, in the body, and not so much in the brain,
so that cells are inhospitable to virus/bacterial reproduction.
Why? Because they work well at 98.6/ 97.0 ish, and poorly at higher,
and at lower,
temperatures.
2. The cytokine storm brought by flu virus vias Sialic Acid receptors
and by Covid, via ACE2 receptors and antigen DNA/RNA presence
Creates: a highly inflammatory chemical neighbourhood.
3. When some crucial, yet still-unknown-cause keeps the Hypothalamus
and the cell
in FEVER mode, and/or in low ATP production mode,
CFS patients suffer needlessly.
WE NEED TO ascertain the cause of the CDR Cell Defense Response
remaining STUCK in the innate immune response,
and not
growing up
and deferring to the Adaptive Immune Response of
Antibody creation and multiplication.
… The fever BUYS TIME for the Antibodies to be created
and to proliferate.
That is our question.
Hopefully:
if we can kill off damaged mitos through mitophagy,
then supply the healthy mitos with Red Light (unpaid)
energy, the new mitos can make a
come-from-behind-victory.
Because… let’s face it:
It was never medicine/medication that healed a cut,
a scrape, nor a bruise, nor that ended your fever as a
child.
It was:
your innate, followed by Adaptive, immune systems.
FEED THEM
Hi Cort,
Thanks so much for this summary of new mitochondrial articles. It’s good to see that the consensus is shifting to include mitochondrial dysfunction as a cause of various aspects of LC.
Trained in immunology at Dartmouth Medical School, I came to agree with the early adopters of the mitochondrial dysfunction (MD) hypothesis about 2 years ago. Since then I’ve been refining my own protocol to help me recover from LC.
Successfully treating MD in LC requires an appreciation for the difference between primary and secondary MD. Primary MD is caused by in-born genetic mutations generally in the mitochondrial DNA. These are specific and regular. Secondary MD is caused by mutations of mitochondrial DNA from environmental, inflammatory and infection associated events. These mutations are more random and can involve many of the genes in the mitochondria.
I would predict that over time, the randomness of secondary mitochondrial mutations will be well established in the scientific literature.
This actually gives an advantage to those of us who are focused on recovering from LC and similar illnesses associated with secondary MD.
The hope lies in the process of mitochondrial biogenesis, the process whereby mitochondria can self-repair and replicate. The bottom line is to stay focused on self-care that can promote that process.
This mean, in short, reducing lifestyle and environmental sources of inflammation while providing mitochondria with the nutrients that are needed for production of ATP and to promote mitochondrial biogenesis.
Details of this process and protocol are freely available on my posts on Substack: https://longcovidjourney2wellness.substack.com/
Thanks again Cort.
Kind regards,
Mardi
WORD !
Mardi,
after 4 + years of trying to understand what causes CFS and what can treat it,
I here recognize your comments as EXACTLY what every follower on this website needs to make their focus.
Things can make a lot of sense to me, or a lot of sense to you,
but that can not make understanding go into a brain.
What I am saying is that your comments are consistent with my much-researched udnerstanding after 4 years and I hope that Cort and others hear.
Hello Christopher D, Cort and fellow readers,
Thank you for your kind words.
If invited, I would be happy to provide a feature article for Health Rising.
I’d be happy to provide a CV to establish my credentials.
As always, I invite readers to read my posts which are largely focused on mitochondrial dysfunction and other factors that cause the symptoms of Long COVID and several other disorders. Subscriptions are freely available.
https://longcovidjourney2wellness.substack.com/
Mardi,
I good faith I followed your link and chose a free subscription.
The next thing I had access to was a free-for-all of public opinion including yours, when purportedly it would be only about your posts. This does not seem to be your profile, but a public discussion board.
Unless you lead me otherwise, I do not see the value in remaining a subscriber there.
Please provide something better. If you need some props on using WordPress, I can help.
Chris
Hi Chris,
I suspect that you received a link to a public grouping of various selections from Substack. I think that Substack may send this sort of thing out to acquaint new subscribers to a wider range of topics.
To go to my Substack site, go to the link previously listed. There should be about two pages of specific posts that I’ve made over the last two years.
I have not posted this last week due to illness in my family. If you finish reading older posts, please be patient. I work hard to write meaningful and accurate posts based on my training, experience and published scientific data. It takes time to do it right.
Thanks for your kindness.
Hi Cort,
Thanks so much for this summary of new mitochondrial articles. It’s good to see that the consensus is shifting to include mitochondrial dysfunction as a cause of various aspects of LC.
Trained in immunology at Dartmouth Medical School, I came to agree with the early adopters of the mitochondrial dysfunction (MD) hypothesis about 2 years ago. Since then I’ve been refining my own protocol to help me recover from LC.
Successfully treating MD in LC requires an appreciation for the difference between primary and secondary MD. Primary MD is caused by in-born genetic mutations generally in the mitochondrial DNA. These are specific and regular. Secondary MD is caused by mutations of mitochondrial DNA from environmental, inflammatory and infection associated events. These mutations are more random and can involve many of the genes in the mitochondria.
I would predict that over time, the randomness of secondary mitochondrial mutations will be well established in the scientific literature.
This actually gives an advantage to those of us who are focused on recovering from LC and similar illnesses associated with secondary MD.
The hope lies in the process of mitochondrial biogenesis, the process whereby mitochondria can self-repair and replicate. The bottom line is to stay focused on self-care that can promote that process.
This mean, in short, reducing lifestyle and environmental sources of inflammation while providing mitochondria with the nutrients that are needed for production of ATP and to promote mitochondrial biogenesis.
Thanks again Cort.
Kind regards,
Mardi
Hi Mardi – Thank you for the information and the link to some of your work. What do you think about fasting and getting into a state of autophagy to aid the mitochondrial repair cycle?
You are ahead of your time.
Do this.
This is what functional medicine is breaking through with
this year/month/ week.
It is only food. Do it.
An absolute tour de force Cort. Bravo. You have a true gift in grasping the research and sharing summaries of them. Thank you for your efforts, you help move the ball forward.
Thanks!
This is a little off topic, but I just got my blood tests prior to seeing my doctor in November. My homocysteine has been consistently high and C02 low. My B12 and folate are normal. I also had an abnormal ANA. Does anyone else have test results like these?
I will answer.. hold me to it.
Hi Cort,
Thank you very much for following the research and presenting it in an easily understandable manner.
I was wondering if you have any knowledge of research into G6PD deficiency in ME/CFS. It is common (7.5% of the population) , causes deficiency in NADPH which impacts energy production, neurotransmitters synthesis, immunity, the brain etc. Ron Davis is currently conducting a research on BH4 deficiency which leads to lack of NADPH and the same symptoms. I just found out I have G6PD gene mutation that causes the enzyme deficiency and am wondering if this is my loaded gun. Infections could trigger the symptomatology which is as a result of increased oxidative stress.
Mayer KP, Ismaeel A, Kalema AG, Montgomery-Yates AA, Soper MK, Kern PA, Starck JD, Slone SA, Morris PE, Dupont-Versteegden EE, Kosmac K. Persistent Fatigue, Weakness, and Aberrant Muscle Mitochondria in Survivors of Critical COVID-19. Crit Care Explor. 2024 Oct 16;6(10):e1164. doi: 10.1097/CCE.0000000000001164. PMID: 39412208; PMCID: PMC11487221.
Can be downloaded here: https://pmc.ncbi.nlm.nih.gov/articles/PMC11487221/
Hi Cort,
This paper reference is off topic but extremely interesting.
This paper from the ”Centre Hospitalier de l’Université de Montréal (CHUM)” talks about a method developed for brain retraining to fix various problems like functional neurological disorder, fibromyalgia, irritable bowel or chronic fatigue. This is a special clinique in the hospital run by a full team of neurologists, neuropsychiatrist, physiotherapist, occupational therapist, etc.
This is in french but you can pass it through google translate. It’s worth the read !!!
https://ici.radio-canada.ca/nouvelle/2113390/trouble-neurologique-fonctionnel-cerveau-programme-chum
Cheers
Thanks!
Hi Cort,
Here is a very interesting video explaining the Functional Neurological Disorder (FND), some reasons that can lead to it and the fact that the brain can be retrained to react differently and often get back to normal.
https://www.youtube.com/watch?v=Kd9CHyDUEPk
”Trouble Neurologique Fonctionnel”
This link is in french but under the video itself, if you expand the gray zone, you can select between english, italian, german, etc versions.
Hello Canada ! It’s Ontario speaking.
I translated to English, thank you, and right away I was hit by a medicine bias:
That’s okay, I’m going to keep reading, with an open mind.
But the person interviewed said “They’re not crazy.”
Great.
No one ever should have thought that they were.
But I am AMAZED by the fact that these various medical specialties
are NOT silo’d in this CHUM. Frankly— for 4 years I have been
muttering to myself that
if ONLY the specialists would intermingle, they could DROP their
biases and ACCELERATE healing. Here it seems possible !!
But I looked up “psychosis”… (in other words: “you’re crazy”)
They may be referring to what we call hypochondria, or to what
we call paranoia– some disease is trying to harm them.
But PYSCHOSIS??? That is completely misguided.
Here is the NIH definition of psychosis:
“People with psychosis typically experience delusions (false beliefs, for example, that people on television are sending them special messages or that others are trying to hurt them) and hallucinations (seeing or hearing things that others do not, such as hearing voices telling them to do something or criticizing them). Other symptoms can include incoherent or nonsense speech and behavior that is inappropriate for the situation.”
Completely not called for from that interviewee.
Did you happen to watch the movie from France called Delicatessen?
Do you remember Aurore? Okay…THAT would be psychosis.
….except… it was real voices.
More to follow in a constructive way, not just damage control.
P.S. EXCELLENT contribution.
Anyone know how this related to histamine dumps that are so prevalent in long covid ?
I will answer.. hold me to it.
How about treatments that reverse the migration of monocytes to muscle to become macrophages? How can these tissue macrophages be studied? How do they react to exercise? Are they signaling nearby muscle mitochondria? How does this process differ when compared to healthy patients or those who recover?
As I mentioned in several forum posts at phoenixrising, very long chain fatty acid is X-linked adrenoleukodystrophy (X-ALD), a condition related to muscle weakness.
It me deserve a closer look:
X-linked adrenoleukodystrophy: ABCD1 de novo mutations and mosaicism
https://pubmed.ncbi.nlm.nih.gov/21700483/