

Geoff’s Translation
GIST
Health Rising recently covered two big complex studies – one from the Lipkin group and the DecodeME genetic studies – and now here’s the BioMapAI study, which uses a very different approach. The authors are very high on this unusual study.
“BioMapAI provides unprecedented systems-level insights into ME/CFS, refining existing hypotheses and hypothesizing unique mechanisms—specifically, how multi-omics dynamics are associated to the disease’s heterogeneous symptoms.”
“This study represents a substantial technical and biological advance over our previous work and other investigations of ME/CFS to date”
“We hope that the unprecedented (there’s that word again) systems-level resolution of our dataset, algorithm, and analyses will contribute to filling out heretofore unknown links between these factors thus explaining some of the disease heterogeneity in this important disease.
Then, again, it’s quite the study. For one thing it’s drenched in “AI” and an innovative AI approach at that.
This study assessed large amounts of data to see if it could linkages between diffferent systems
The authors state “We further introduce an innovative AI approach that begins to address the multifaceted nature of this chronic disease”. They emphasize that the study is not about finding the cause – it’s about understanding ME/CFS as it exists right now in the patients’ bodies. This study threw immense amounts of data together to valid prior findings and generate “unique hypotheses” that should be explored further.
Addressing the “multifaceted nature” of ME/CFS is, of course, the holy grail. ME/CFS has so many moving pieces that perhaps the great question in this disease is how to put them together, which brings us to AI. Finding patterns that no one else can see is one thing AI is really good at.
The study was funded by a series of NIH grants to Julia Oh (first senior author) and Unutmaz (second senior author). The authors also included Suzanne Vernon and Lucinda Bateman from the Bateman Horne Center.
It was good to see Suzanne Vernon -a pioneer in this area – on this study. Back in 2006, she produced one of the first large efforts in any disease to attempt to integrate large amounts of data together (epidemiological, clinical, laboratory, and gene expression data) to build a disease model for ME/CFS. (One of the papers resulted in Wang’s muscle findings).
That was almost 20 years ago, and how things have changed. This 249-person, 4-year study incorporated gut metagenomics, metabolomics, immune cell profiling, blood laboratory data, and clinical symptoms. The study was able to accomplish what few other studies have – follow a large number of participants (154 ME/CFS, 96 healthy controls) over a 3-4 year period.
The GIST
-
The effects gut bacteria have are at the heart of this paper.
The BioMapAI paper presents an innovative and unique AI approach that begins to address the multifaceted nature of ME/CFS. The 249-person, 4-year study incorporated gut metagenomics, metabolomics, immune cell profiling, blood laboratory data, and clinical symptoms. It assessed how metabolic pathways in the gut affect the metabolites, immune system, etc.
- A broad dysregulation of the adaptive (later) immune response involving B and T-cells appears to be present
- Instead of protecting the gut, the gut flora or microbiome in ME/CFS appears to have triggered immune cells to attack the gut lining.
- Activation of the kynurenine pathway in ME/CFS was associated with cytotoxic and inflammatory immune cells attacks on the gut lining. The lipid (fats) in ME/CFS appear to be associated with gut lining attack as well.
- Reduced butyrate and branched chain amino acid levels suggest that the gut environment in ME/CFS has been shifted from an energy-secure, Treg-protected one to an energy-stressed, Th17-skewed, inflammatory one.
- A key gut /liver interaction has come undone in ME/CFS, resulting in impaired lipid metabolism, and possibly damaged cellular membranes, more reactive oxygen species (free radicals), and problems with mitochondrial function. functioning.
- As time goes on, the disease appears to become more entrenched, with more biological pathways becoming dysregulated.
- For your entertainment….ChatGPT 5.0’s analysis of findings from this paper, the recent Lipkin paper, and the DecodeME paper suggest that the findings cohere and support each other.
- ChatGPT 5.0 proposed that, based on these three papers, the top three issues driving ME/CFS are butyrate depletion in the gut, reduced acylcarnitines (low energy) and impaired redox (antioxidant system), which both cause and amplify the energy problems.
Dr. Ruoyun Xiong created the BioMapAI – an “explainable supervised deep neural network (DNN)” program. Deep neural networks use many “hidden layers” to capture complex relationships that would otherwise be missed.
The authors stated “This study generated among the most extensive paired multi-omics datasets for ME/CFS to date”. The paired part is the key. Most multi-omics data sets pool genomics, metabolomics, etc. data from many individuals and then attempt to assess the connections between them.
In contrast, paired multiomics studies assess all the “omics” data from each individual first, and then compare the findings of that individual to those of other individuals. Using pairing, you could determine, for instance, if a change in the expression of a gene results in the production of the protein that gene makes or if a metabolic shift within that individual results in the increased production of inflammatory markers.
Ultimately, pairing is a much more precise and powerful way of doing “systems biology”; i.e., understanding how the different systems of the body are working together.
The study aimed to identify biomarkers associated with symptoms and create maps that illustrate how the gut, immune, and metabolic systems interact in ME/CFS.
The analyses they used are far, (far), beyond my understanding. Suffice it to say it went far beyond most machine learning projects, that it followed a lot of people over 4 years, and assessed a large amount of gut, immune, metabolomic, and immune cell data to see if they could build a comprehensive model of ME/CFS.
Gut Bacteria
The effects gut bacteria have are at the heart of this paper.
The effects gut bacteria have on other systems are at the heart of this paper. The researchers started by identifying harmful microbial/microbiome/gut networks. Then they mapped the metabolic pathways found in them to plasma metabolites and immune cells.
It makes sense to start at the gut as it (and the liver) are the first places where food and toxins and processed and where many metabolic pathways begin.
Notice how familiar the most prominent metabolites (short-chain fatty acids, tryptophan catabolites, bile acid derivatives, etc.) produced by the gut bacteria are. The fact that short-chain fatty acids, tryptophan catabolites, and bile acid derivatives have all been implicated in ME/CFS suggests that gut issues are having a real impact.
One-Two Punch
The key with these big “omics” studies is to validate a finding by having it show up in two different areas. For instance, in this study, the researchers identified the metabolic pathways active in harmful gut networks and then examined whether the metabolites or immune factors associated with those pathways were present. The metabolites and immune findings, in other words, are the reality check. Once you get that one-two punch you know you’re onto something.
Results
Please note that I used ChatGPT extensively in this blog. I asked it to explain the findings in italics from the paper below.
Based on BioMapAI’s predictions and subsequent network analyses, we propose that some of the disease-specific changes in ME/CFS arise from disrupted associations between the gut microbiome, immune system, and metabolome. The authors
Note that it’s not the gut microbiome or the immune system or the metabolome – it’s the broken/pathological associations between these systems that are, at least, in part, driving these diseases. In the end the systems are just not interacting properly.
“Broad dysregulation of the adaptive immune response”.
Dysregulation of the immune system – particularly the B and T-cells – was associated with many symptoms.
It was interesting to see the immune cells take precedence, as the study was very gut-oriented. This is the only finding, though, that’s solely focused on immune cells.
Increased B cells (CD19+ CD3−), CCR6+ CD8 memory T cells (mCD8+ CCR6+ CXCR3−), and CD4 naive T cells (nCD4+ FOXP3+) in patients were associated with most symptoms, suggesting a potentially broad dysregulation of the adaptive immune response.
At this point, it’s somewhat challenging to identify an immune cell that hasn’t been associated with ME/CFS; however, the B and T-cells have certainly been leaders. The authors stated that “a broad dysregulation of the adaptive (later) immune response” is potentially present.
This suggests that a) chronic immune activation is present and that b) an attempt is also being made (FOXP3+) to rein the system in. This type of pattern typically occurs when chronic immune activation – when an infection, autoimmune response or some other type of stimulation – is present.
This isn’t a new finding but it does highlight the central role B-cells may be playing in this disease.
Altered Associations
We begin to see healthy connections breaking down…
“Healthy control-derived interactions, such as the microbial pyruvate module associating with multiple immune modules, and connections between commensal gut microbes (Prevotella, Clostridia sp., Ruminococcaceae) with Th17 memory cells, plasma steroids, phospholipids and tocopherol (vitamin E) were disrupted “
Instead of maintaining homeostasis, the gut bacteria in ME/CFS are enhancing inflammation.
In healthy people, interactions between gut microbes and the immune system, steroids, lipids, etc., help to maintain homeostasis and health. Those interactions or connections have come undone in ME/CFS.
The broken pyruvate interaction may be particularly telling since it affects two metabolites that appear to play an important role in ME/CFS: butyrate and small-chain fatty acids. Pyruvate should also regulate the TH17-Treg interaction, which we’ll see has been upended in ME/CFS as well.
The commensal gut bacteria (Prevotella, Clostridia sp., Ruminococcaceae) normally affect steroid hormones, the lipids, and regulate the immune system so that it protects the gut lining but does not injure it.
That’s not happening in ME/CFS. Instead of protecting the gut lining, these gut bacteria appear to be having the opposite effect resulting in more damage to the lipids and the gut lining resulting in fatigue, mood and sleep issues.
A Profound Imbalance
Increased correlations between gut microbiome and mucosal/inflammatory immune modules, including CD8+ MAIT and IFN-γ+ CD4 memory cells, suggested an increased association with microbiome and inflammatory elements in ME/CFS
Hyperactive T-cells appeared to be attacking the gut lining. (T-cell in blue)
The gut microbiome in ME/CFS overall was associated hyperactive CD8+ MAIT and IFN-γ+ CD4 memory cells. MAIT cells are especially abundant in mucosal tissues lining the gut and lungs. When properly activated, they provide a first line of defense against bacteria and fungi and also protect against viruses. When overly activated, though, they can produce inflammation and have been associated with autoimmune diseases.
Again, it appears that the microbiome in ME/CFS has turned these formerly protective cells into purveyors of inflammation and possibly autoimmunity. Plus, in Unutmaz’s explanation, below, we see another connection that is going to pop up several times in this study – tryptophan. From Derya Unutmaz
“MAIT cells bridge gut health to broader immune functions. Their disruption alongside butyrate and tryptophan pathways, normally anti-inflammatory, suggests a profound imbalance.”
A Central Hub? The Tryptophan Pathway Goes South
“For example, increased tryptophan metabolism, associated with gastrointestinal issues, lost its negative association with Th22 cells, and gained correlations with γδ T cells and the secretion of IFN-γ and GzA from CD8 and CD8+ MAIT cells.”
The recent Lipkin group paper highlighted the tryptophan pathway – and here it shows up in a microbiome oriented study. Because tryptophan is considered a “hub connector” that potentially links gut, immune and mitochondrial pathways together a disruption in this pathway could play a core role in this illness.
This study found that tryptophan metabolism was directed down the inflammatory and neurotoxic kynurenine pathway (and away from serotonin) in the ME/CFS patients.
Because Th22 cells play an important role in defending the gut lining, the loss of the normal association with the tryptophan pathway means more problems with the gut lining….
That’s an expected outcome with kynurenine pathway activation, but in ME/CFS patients, something extra is added. The aberrant tryptophan pathways are also associated with cytotoxic and inflammatory immune cells attacking the gut lining; i.e., an already bad situation just got worse. This scenario could result in chronic gut inflammation, leaky gut, and ultimately, as the gut materials leak into the bloodstream, systemic inflammation.
Plus, this pattern could also result in NAD+ depletion and a disruption in the TCA cycle which feeds the electron transport chain (oxidative phosphorylation)
Gut Lining Under Attack Again
“Plasma lipids had more correlations with γδ T and CD8+ MAIT cells”
The microbiome in ME/CFS patients appeared to have turned immune cells to attack the gut lining.
If that wasn’t enough, we saw more correlations pop up with these bad immune actors. It appears that the lipids (fats) found in ME/CFS patients’ plasma are also triggering immune cells to attack the gut lining. This study suggests both the tryptophan pathway in the gut and lipids in the plasma help trigger an attack on the gut lining.
Energy Stressed Gut Environments
“Networks associated with emotional dysregulation and fatigue exhibited decreased butyrate production and BCAA biosynthesis and opposite correlations with Th17 and regulatory T cells (Treg cells) in the ME/CFS patients”
From the first gut metabolomics studies in ME/CFS, low butyrate levels have stood out. We generally think of butyrate as a key gut lining protector, but it also supports the mitochondria and the antioxidant system.
The decreased branch chain amino acids (BCAA) feed the Krebs/Citric/TCA cycle (which then feeds the oxidative phosphorylation, which produces ATP). Low BCAA levels, then, can result in reduced energy levels and fatigue.
The reduced butyrate/BCAA levels seen in the ME/CFS patients’ guts are also, as the prior findings were, associated with immune changes that enhance inflammation. They suggest that the gut environment in ME/CFS has been shifted an energy-secure, Treg-protected one to an energy-stressed, Th17-skewed, inflammatory one.
An important gut/liver interaction was off.
Gut/Liver Network Flipped?
“Additional health-associated interactions between microbial benzoate metabolism and various plasma metabolites (for example, lipids, glycerophosphoethanolamine, fatty acids, and bile acids) we hypothesized are also diminished or reversed in ME/CFS”
The benzoate produced by gut bacteria is turned into hippurate in the liver. Hippurate “improves” lipid metabolism, resulting in increased cell membrane integrity, mitochondrial function, and circadian/metabolic tone.
That doesn’t appear to be happening in ME/CFS, though.. With hippurate no longer contributing to lipid metabolism, however, damaged cellular membranes, more reactive oxygen species (free radicals), and problems with mitochondrial functioning are likely to show up. Indeed, we have already seen lipid problems pop up.
Specific Gut Bacteria
“The species model highlighted the correlation of Dysosmobacteria welbionis and butyrate metabolism. The metabolome model categorized increased levels of glycodeoxycholate 3-sulfate, a bile acid, and decreased vanillylmandelate, a catecholamine breakdown product”
Dysosmobacter welbionis J115T turned out to be an important gut bacterium, indeed. Here, we have a notable link between a reduction in gut bacteria in ME/CFS patients and an accompanying alteration in bile acids, as well as two metabolites (butyrate and vanillylmandelate).
Reductions in Dysosmobacter welbionis J115T tie in with the reductions in – there it is again – butyrate, as well the inflammatory immune changes we’ve seen (reduced Treg/ increased inflammatory Th17), as well as increased gut barrier “fragility, and, once again, increases in reactive oxygen species.
The increased levels of the bile acid could tie up CoA/ATP – producing more stress on the mitochondria, and the decreased catecholamine levels could translate into gut–brain/autonomic dysregulation.
The Faecalibacterium prausnitzii dilemma
“A biphasic relationship was observed in the association of Faecalibacterium prausnitzii with pain, whose saddle curve had a mixture of positive and negative contribution peaks meaning that either abnormally low and high relative abundances could be associated with pain severity.
Things got more complicated when it came to F. prausnitzii – a key butyrate producer. Higher F. prausnitzii levels should result in more butyrate, tighter gut junctions, a more balanced immune system, and less pain, but in ME/CFS, higher F. prausnitzii levels were associated with more pain.
This could be caused by several reasons. FP levels in the fecal matter might be high, while those in the gut lining might be very low. FP “blooms” in an unbalanced gut might throw other parts of the gut off, or FP might look high relative to other bacteria, but if the gut microbiome is really off, it might not be. ‘
In any case, it appears that there’s a sweet spot for F. prausnitzii in ME/CFS; neither too high or too low.
Duration Matters
While the study was only 4 years long it suggested that as time goes on the disease state deepens and more breakdowns occur.
“Our data indicate these biological disruptions become more entrenched over time. That doesn’t mean longer-duration ME/CFS can’t be reversed, but it may be more challenging.” Dr. Unutmaz:
Solid Findings
The senior lead author, Dr. Oh, pointed out the findings were solid.
“Despite diverse data collection methods, common disease signatures emerged in fatty acids, immune markers, and metabolites, That tells us this is not random. This is real biological dysregulation.”
The team plans to make their dataset and BioMapAI platform widely available.
Overview

This study was about opening up new arenas to explore.
Studies like this don’t provide answers so much as new arenas to explore. This study could be providing answers; i.e., it could be describing interactions that make a huge difference in ME/CFS. We just won’t know until the findings are tested.
For now, they’ve dug more deeply into the interactions the microbiome has with other systems in ME/CFS than anyone has before.They indicate that the gut environment in ME/CFS and how it interacts with other systems has been dramatically shifted.
Instead of being protective, the gut microbiome in ME/CFS is now promoting inflammation and negatively impacting factors that play a role far beyond the gut, such as the steroid hormones, lipids, and mitochondrial functioning.
The microbiome, the lipids, the tryptophan pathway, and the reductions in Dysosmobacter welbionis J115T all appear to be triggering inflammatory immune cells to attack the gut lining.
Decreased butyrate and branched-chain amino acids suggest that an energy-stressed, inflammation-skewed gut environment is present.
The odd finding – that higher levels of the protective bacteria F. prausnitzii are associated with more pain – indicates how discombobulated the gut environment has become in ME/CFS.
The fact that the breakdowns in the microbiome-metabolic networks were associated with more severe fatigue, emotional disturbances, and sleep problems suggests that the microbiome problems in ME/CFS are affecting the brain.
Many of these present unique linkages that have not been seen before and can be tested.
Treatment
Treatment suggestions were general and included tailored probiotics, anti-inflammatory therapies, immune-modulating drugs that target the hyperactive immune cells, or diet interventions guided by specific microbiome and metabolome profiles.
Connections, Connections…the Lipkin Group’s Recent Paper
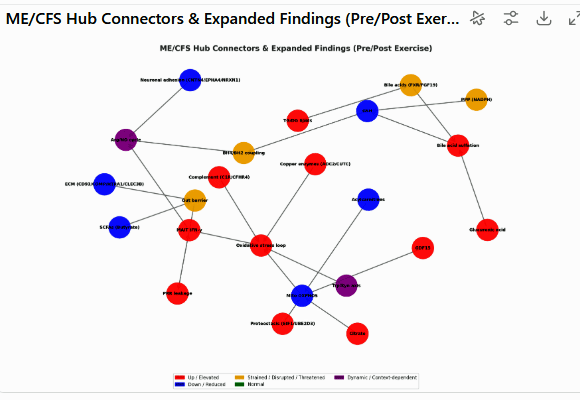
Possible connections given data from the Oh and Lipkin group’s study – courtesy of ChatGPT 5.0. Purple = Dynamic (changes pre→post exercise, e.g., Trp/Kyn, ORN:CIT ratios).
Red = Elevated, Blue = Reduced, Orange = Strained/Disrupted/Threatened, Green = Normal.
For your entertainment! Next, I asked ChatGPT how these findings from this paper interacted with the findings of the recent Lipkin group’s study. Whether all of this is correct is way, way, WAY beyond my pay grade. I simply present it’s findings as interesting data points.
The two studies differ significantly: one focused on the microbiome’s interactions with various systems, while the other assessed metabolomic, proteomic, and immune cell activity before and after exercise and immune stimulation. A finding that the results of these studies complement each other would be a good thing, given their very different thrusts.
- Immune Findings – ChatGPT 5.0 believes that the immune findings from the Lipkin study (↑ CXCL5, GM-CSF, IL-1β, IL-2, IL-6, IL-8, IL-23, IFN-γ, IL-13, IL-17, TNF-α (baseline) → PRR→IL-12/IL-18 priming (#17) and Oxylipin–cytokine axis (#18)) support the Oh study findings of a Th1/Th17-skewed (pro-inflammatory) mucosal-innate program associated with gut lining attack, MAIT T-cell activation and reduced butyrate.
- Extracellular Matrix (Connective tissue) – the Lipkin study’s finding of ECM/vascular remodeling fragility is consistent with the problems of intestinal barrier integrity found in the Oh study
- Tryptophan/Kynurenine pathways – both studies suggest that kynurenine pathway activation and accompanying inflammation
- Acylcarnitines/Butyrate connection: the low acylcarnitines and reduced mitochondrial activity in the Lipkin study align with the Oh study’s finding of low butyrate levels and a system under systemic energy stress
- Lipids – both papers suggested that problems with lipid handling/transport exist. The Oh paper found that normal interactions between the microbiome and lipids are gone in ME/CFS, while the Lipkin group’s paper found dysregulated levels of triglycerides/acylcarnitines.
The Decode ME GWAS Study?
Next, I asked ChatGPT 5.0 to add in results from the Decode ME/CFS GWAS genetic study – leaving us with a model that incorporates the microbiome-oriented study, the proteome/metabolome/immune exercise study, and a genetic study. I asked ChatGPT 5.0 whether the findings from the GWAS study “contribute or not to our model”?
The analysis did not find anything to contradict the model being built. If anything, its findings appeared to enhance the innate immune hyperactivity and “trained immune responsiveness” found, the problems with “barrier integrity” (aka intestinal barrier), One finding (chr6p22.2) was labeled a “great fit” to immune findings from both the Oh and Lipkin studies. Another”gene significant loci” fit well with the Lipkin studies’ finding of post-exercise nervous system dysregulation.
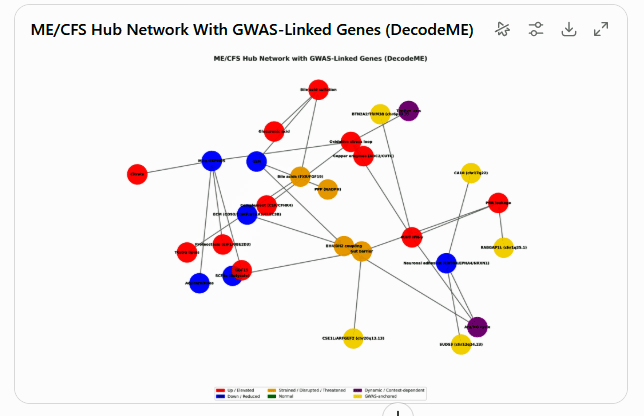
ChatGPT model of Lipkin/Oh/DecodeME results
That’s pretty complicated, so I asked it what it thought were the hottest, i.e., most determinative nodes of its model? In other words, what did these three studies suggest was most likely to cause this illness?
It proposed that three top tiers were present.
- Butyrate depletion in the gut produced both the gut barrier leak + mucosal immune skew. Without restoring SCFAs, the cascade self-perpetuates.
- Reduced acylcarnitines under-fuels oxidative phosphorylation, resulting in the GDF15, citrate, impaired phosphate handling sets “the stage for post-exertional collapse” and the lipid problems.
- Impaired Redox (antioxidant system) – both causes and amplifies the energy + immune dysregulation.
To wit:
the “engine room” looks to be butyrate–barrier, FAO–OXPHOS fuel mismatch, and redox buffering. The GWAS genes suggest that who “tips over” into illness depends heavily on innate-immune priming (RABGAP1L/BTN2A2) and brain circuits (SUDS3/CA10).”
I have no idea if this is correct, and it’s based on the results of just three studies, and many more studies assess other parts of ME/CFS are out there… For your entertainment only.






Ive always thought that if I could give my gut a break by feeding the body via intervenous it would give the gut time to heal on its own.
I proved this by fasting in the early stages of my illness but I later fell apart and ive been stuck ever since.
Then, years later I find a few peeps that in fact cured their illness by fasting