

The IACFS/ME Zoom 2020 Conference may have been a short 1-day affair, but it featured some really intriguing presentations. Health Rising reported on some startling results from Marvin Meadow’s oral rehydration studies earlier.
The Immune Hole That Produced ME/CFS? (and who knows – maybe long-hauling as well)
Somehow Lenny Jason got a hold of some recovery data from the 1918 flu! The data suggested that 20% of those affected may not have fully recovered, and fatigue was one of the most common consequences. Jason pointed out that fatigue is a common result of post-infectious illnesses. Almost 30% of those who survived the Ebola virus, and over 40% of those who survived the first (and more deadly) SARS virus reported severe fatigue.
An ME/CFS researcher – and, in particular, this researcher – would know. More data on post-infectious illnesses have come out of ME/CFS research than any other disease, and Jason has been following a post-infectious cohort for years.
First there were the groundbreaking Dubbo studies. Then came the Taylor/Jason/Katz effort to follow 301 adolescent patients with infectious mononucleosis (IM) for several years. They found 13% met the criteria for ME/CFS at 6 months, 7% at 12 months and 4% at two years.

Now, Jason and Katz are presently in the midst of what appears to be the largest post-infectious illness study ever. This study, which began 7 years ago, has been tracking 4,500 college students. The goal – to wean out those who come down with infectious mononucleosis/glandular fever, and to determine why some came down with ME/CFS. It’s been a massive effort.
They found that about 5% of college students (@200) came down with IM. Of those, eight percent still had severe symptoms at 6 months.
Jason’s study is unique in that he collected samples from the students before they became ill with IM, and eventually ME/CFS. That meant he had the opportunity to assess factors which opened the door to ME/CFS.
Everyone who has come down with these illnesses has wondered: what happened? Why me? This is the first time, that I know of, that a study has the potential to answer these questions.
The Immune Hole That Caused ME/CFS?
Jason reported that his study revealed a possible immune hole which may have allowed people with IM to progress to ME/CFS. The study – which has been submitted to a journal – found reductions in three immune factors: IL-5, IL-6 and IL-13 – in the college students who went onto develop ME/CFS.
- Interleukin 5 (IL-5) – stimulates B cell growth and enhances the secretion of immunoglobulins/antibodies. IL-5 has a protective aspect, as high IL-5 levels enhance the production of B1 cells which are anti-inflammatory. Impaired B1 cells have been found in multiple sclerosis, systemic lupus erythematosus and rheumatoid arthritis.
- Interleukin 6 (IL-6) – IL-6 can be both pro and anti-inflammatory. As an anti-inflammatory myokine, it reins in one of the nastiest cytokines of all – TNF-a. If low IL-6 levels are failing to rein in TNF-a during an infection, bad things could surely result. Interestingly, levels of the IL-6 myokine rise dramatically during exercise.
- Interleukin 13 (IL-13) – Secreted by many immune cells (T helper type 2 (Th2) cells, CD4 cells, natural killer T cells, mast cells, basophils, eosinophils and nuocytes), IL-13 is best known to produce inflammation associated with allergies. Like the other cytokines that popped up in Jason’s study, though, IL-13 also has anti-inflammatory properties. It’s not clear how low IL-13 levels might play in the mix, but low IL-13 could make it more difficult to repel parasites.
Could Jason have uncovered the immune hole which got things going in ME/CFS? (Image-by-dric-from-Pixabay))
That’s potentially big news. No one, to my knowledge, has been able to identify a possible immune hole in people who were healthy and then went on to develop ME/CFS. This is not the end of the analysis. Individual cytokines can only tell us so much. Cytokine network analyses, on the other hand, can show how entire immune networks have been shifted. Jason reported that he will next do cytokine network analyses.
That’s just the beginning of Jason’s post-infectious work, though. Next, Jason will analyze samples taken after the students came down with IM to see if he can identify key changes which directly led to an ME/CFS state.
ME/CFS isn’t the only post-infectious illness around, though. When COVID-19 hit, upon re-contacting the students, Jason found that about 5% of them had become infected with the coronavirus. Now he’s doing the same thing with them that he did with ME/CFS: he’s analyzing their baseline (before illness) samples to see if he can find clues to why some of them became long haulers. Then he’ll assess their post-infectious samples to dig for more clues as to why some of them are not recovering.
Jason, then, is now able to do two unique things: a) he’ll be able to look for immune holes that were present in those who came down with ME/CFS after either post-infectious mononucleosis or COVID-19; b) then he’ll try to track down what changed as they became ill. Plus, he’s doing this in the same cohort of original participants. If all goes well, he’ll be able to report to us if the long haulers have the same (or different) susceptibilities to ME/CFS that ME/CFS patients who had infectious mononucleosis do, and he’ll be able to determine if their diseases progress in the same way. Jason will be following the COVID-19 group for several years.
It’s a fantastic opportunity that was set up by Jason’s decision to focus on post-infectious illness seven years ago. It’s no surprise that an ME/CFS researcher has a leg up on the other COVID-19 researchers. This has been a topic of major interest in ME/CFS for years.
Jason noted that a large Body Politic COVID-19 Support Group survey indicated that 90% of those who took the survey had not recovered after 40 days.
It’ll be fascinating to see if a similar “immune hole” shows up in the infectious mononucleosis ME/CFS group and the COVID-19 long haulers, or if something different is at work with another type of infection.
Jason is looking for children and adolescents with ME/CFS patients and COVID-19 as well as healthy children and adolescent who’ve come down with COVID-19 to provide him data on symptoms and disease progression. Find out more about the survey here and pass it around.
Q and A Period
During the Q&A, Leonard Jason pointed out (once again) that we are a field – maybe the only field – that doesn’t have a validated case definition. That problem results in incredible heterogeneity in our samples (the wastebasket diagnosis problem) – which, of course, skews findings and makes researchers and drug companies loathe to enter this field. Jason believes our inability to find markers is probably in large part due to our inability to create a solid case definition.
He was also asked how aggressively an ME patient should pursue a mold diagnosis. He replied that mold is a *critical factor for ME* and it could be a predisposing factor for COVID.
Starving for Energy? The Norwegian ME/CFS Studies
Another year and another Norwegian researcher digging into the energy problems in ME/CFS. Just last year, Dr. Katarina Lien published a groundbreaking study demonstrating that people with ME/CFS are indeed producing increased levels of lactate (a toxic byproduct of anaerobic production) during exercise in ME/CFS. Now, here comes Dr. Ina Katrine Petterson, another Norwegian researcher focused on energy production (or the lack of it) in this disease.
Dr. Petterson is from the University of Bergen and is closely with the University of Haukeland – apparently with Fluge/Mella’s Haukeland ME/CFS team – which is not a small team at all. You might think that Fluge and Mella had gone to greener pastures after their long term exploration of Rituximab in ME/CFS didn’t work out – but not at all.
Just this year they’ve published two studies on ME/CFS (a successful open-label cyclophosphamide trial, an HLA allele study – which pointed at immune abnormalities) and a study on cancer fatigue (which found low levels of tryptophan in the fatigued group. They published three more studies last year. The Fluge/Mella ME/CFS team is actually more prolific than ever.
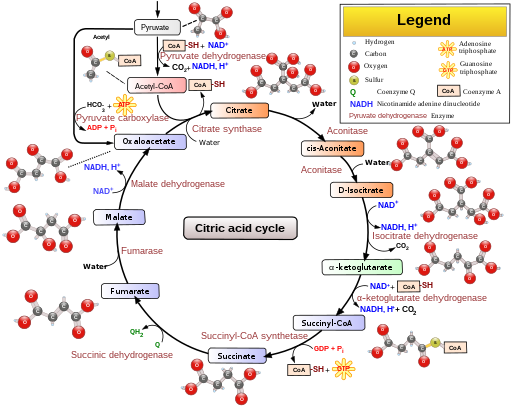
In a nice big study, Dr. Petterson assessed the amino acid metabolites in 255 participants (153 – ME/CFS, 102 – healthy controls). In the central energy pathway, in a process called glycolysis, glucose is first transformed into pyruvate (and a little ATP) in the cytosol of the cell.
Pyruvate is then transformed by pyruvate dehydrogenase (PDN) into actetyl CoA in the mitochondria. Acetyl CoA then enters the TCA cycle, and when all is done, out pops a lot of ATP.
Where the problems in this two-part energy cycle (glycolysis/mitochondria) are occurring – if they are occurring – in ME/CFS is the big question. As several other studies have found, Peterson found no evidence of impaired glycolysis; i.e. glucose appeared to be catabolized into pyruvate in normal amounts.
Next amino acids (lys, leu, try, phe, tyr.) needed to be catabolized into acetyl-CoA in order for the energy production pathway to proceed. Here, Peterson found a breakdown – lower levels of amino acids associated with this pathway than expected. This was important, as an inhibition of the PDH enzyme can lead to reduced energy and increased production of lactate – which was recently found in ME/CFS.
She also found lower levels of amino acids (called anaplerotic amino acids) necessary for the production of the TCA cycle.
Next, she looked at gene expression to try to explain the PDH inhibition – and it did. The expression of genes associated with PDH enzyme inhibitors called PDKs (pyruvate dehydrogenase kinases) was increased. It appears that the PDKs were preventing pyruvate from being catabolized into elements needed to keep the TCA cycle running at full blast.
That meant the cell had to compensate – find other factors to keep the TCA – or aerobic energy cycle – functioning.
To test that hypothesis the team developed cell lines featuring increased PDK expression and then assessed the metabolites the cell lines produced. They found, as expected, that increased levels of fatty acids were being broken down to feed the aerobic energy production cycle. Other studies have had similar results.
Intruder in the Blood

Something in ME/CFS patients blood appeared to be causing the mitochondria to work extra hard – and produce more lactate.
Next came a really fascinating part. Some evidence from Fluge and Mella’s team, and Ron Davis’s team, has suggested that something in the ME/CFS patients’ blood is interfering with energy metabolism. Next, Peterson’s team placed muscle cells into the serum from ME/CFS patients and the serum from healthy controls for six days.
Then they used the Seahorse machine to assess glycolytic functioning and mitochondrial respiration in the cultured muscle cells. Again glycolysis was fine but they found increased – not decreased – mitochondrial production in the muscles cells exposed to the ME/CFS serum. They hypothesized that the muscle cells were reacting to something negative in the ME/CFS patients’ blood. That reaction, though, was not going well. When exposed to “energetic strain”, the muscle cells exposed to the ME/CFS serum were producing higher levels of lactate – a toxic substance.
Starving for Energy?
Lastly, Dr. Petterson suggested that the energy production picture in ME/CFS was akin to what is seen during starvation: glucose utilization drops, ketone bodies soar and fatty acid levels rise.
What could be causing this? An immune response, producing metabolic stress, that is locking people with ME/CFS into a kind of metabolic starvation mode – a finding that smacks of Naviaux’s Dauer or hibernation hypothesis for ME/CFS. The energy production problems in ME/CFS are not homogeneous, though: another study suggested that several metabolic subgroups exist.
Genes
Dr. Petterson also reported that both genetic and metabolomic studies are underway. The Bergen group is clearly rocking.
Takeaways
Jason’s decision to collect samples before the college students came down with mononucleosis paid off when he discovered that the students who came down with ME/CFS appeared to have an “immune hole”; i.e. lower levels of three cytokines.
COVID-19 came at just the right time for Lenny Jason. The cohort of 4500 students he ready to study when the pandemic hit – is allowing him to do something very unusual -trace the development of two post-infectious illnesses, in the same cohort no less, at the same time.
Jason will be analyzing samples taken at baseline – before the students became ill – and as they become ill in an attempt to understand the immune changes taking place in after both infections.
Jason, then, may be able to help answer one of the biggest questions in ME/CFS and in COVID-19 – why one person recovered when others did not.
Dr. Katrina Petterson of Norway reported on a large effort that is taking place in Norway to understand the energy problems in ME/CFS. Petterson’s analysis of amino acids suggested that glycolysis – the first part of the energy production pathway – is doing just fine. When it comes time to catabolize pyruvate to acetyl-CoA – in the second part of the pathway which takes place in the mitochondria, though, things are going off-kilter.
It appears that an inhibitory enzyme called pyruvate dehydrogenase kinase is interfering with the catabolism of pyruvate. That’s forcing the citric acid or TCA cycle to compensate by breaking down fatty acids – a less efficient substrate – something that is seen in starvation. Petterson proposed that some sort of immune response that is causing metabolic stress is responsible.
Using ME/CFS serum and cultured muscle cells Petterson also helped to validate one of the most intriguing possible aspects of ME/CFS to show up in recent years: the idea something in ME/CFS patient’s blood is causing problems. That something appears to be causing the mitochondria to work extra hard producing more lactate – a toxic substance.








Increased lactic acid production in people with ME/ CFS was discovered by the University of Newcastle (Australia) more than 20 years ago.
Also, this recent study that reports “an alternative fuel source” being fatty acids is contradictory to the study recently in “Health Rising” that reported Amino acids were the alternative fuel source.
What to believe? ?
With regards to fatty acids vs amino acids I don’t know. She described several studies – metabolite, metabolomics and gene expression.This metabolite study used amino acid analyses. The end conclusion – I think I got it right – was that fatty acids were probably being used as energy substrates. The key takeaway, though, I think is that both studies are finding that alternative, less efficient substrates are being used for energy production.
A 1998 study found that increased lactate in response to exercise (of a sort) but was done using needle biopsies – https://pubmed.ncbi.nlm.nih.gov/9527150/. A 2002 study which employed exercise did not find increased lactate. https://pubmed.ncbi.nlm.nih.gov/11782647/ and yes, apologies to the Aussies – I see an Australian study in 2004 did find increased lactate during exercise. https://pubmed.ncbi.nlm.nih.gov/15595287/. It took awhile but I guess their results have been validated!
I was prescribed amino acids years ago from Newcastle University (Australia) . They had no effect. I tried glyconutrients with good effect! The only other thing that has helped me is DHEA. Endocrinologist found low cortisol and low blood sodium.
Cort, if fatty acids are used instead of glucose for energy metabolism, would that not mean that a keto diet (high fat, low carb) is beneficial for us?
To my thinking it suggests that it could help. It would not eliminate the problem but I would think that a low carbohydrate, higher fat and protein might be helpful. I know I don’t do well with carbs and reducing them does help a bit but unfortunately is not a home run. Still anything that helps is good and I’m maintaining it.
I have brain fog, so consider the following with caution. In the context of CFS, PDK enzyme production is upregulated; in order to understand metabolism during upregulated PDK, they developed cell lines with innate upregulated PDK, and found those cells using increased fatty acids. It is not clear whether those new cells were in CFS serum or not; FA utilization might be the most efficient energy method, and thus used by these cells in normal serum (and in vitro), but in CFS serum (or in vivo, with the low O2 due to poor capillary flow hypothesized in CFS) that process may be prevented – thus, in CFS, cells are forced to Plan C: burning amino acids.
Thanks Feral boy! That’s where the fatty acids came from. 🙂 It’s actually in the blog – which I forgot about – which is no surprise at all. My brain gets full and things – lots of them – slop out. 🙂
I was just going to reply along a similar line, but in greatly simplified terms.
Problem 1: when energy demands increase without a properly functioning Citric Acid Cycle, the body cannot make ATP for the working cells at a fast enough rate.
Problem 2: metabolism of fats, which are typically the secondary substrate in energy production, requires both glucose and oxygen. However, the low oxygen levels found in CFS mean that the already impaired CA cycle is further impaired. Fat can be used when glucose levels are low, but the incomplete breakdown is the cause of the increased fatty acids.
Problem 3: This leaves only protein as an energy source, but using protein as a fuel results in increased lactate levels within the muscles. Normally, this is a temporary problem that individuals recover from within 24 hours or so. However, oxygen is required to return lactate levels to normal…But then we are back to the issue of being low in oxygen, so we are stuck.
thanks cydne! i needed the simplfied version!
Cynde- Thanks for your explanation. It was very useful. Quick question on Problem no 2 sections in your writeup. You said “Fat can be used when glucose levels are low, but the incomplete breakdown is the cause of the increased fatty acids.”. I thought Fatty acids are good and hence increased fatty acids should be good too. I Will be thankful if you can clairfy
Thanks as always Corr.
I’ll be watching developments around the metabolism of fats with interest: a treadmill test revealed a total inability to metabolise fats – I can burn only carbs apparently. I wonder whether this is a common manifestation of cfs? Or a cause?
I was convinced I’d found my silver bullet in the amino acid L-carnitine. I used it for many months before I became sure it was supporting my brain function. But I’m back to zero having recently observed it doing nothing I can feel.
You know you’ve hit the jackpot when drug companies won’t enter the space.
Thanks Cort. You’re a legend.
Could this be the reason for high cholesterol levels?
Paula:
Interesting. My blood chemistry is normal in every respect – other than very high cholesterol.
Good thinking.
Lee, this is really interesting. if that (inability to metabolise fats) is common to ME/CFS patients, I assume trends such as the keto diet would have the opposite effect to that desired. i.e. you would gain weight
is that right? (its entirely possible I’m off base)
Kirsten:
Well that’s a bummer. Exertion intolerance prevents me exercising so I’m using a low carb regime to control my weight. Hadn’t made that possible connection. Thanks.
This metabolic defect is common in cfs as I understand.
That was my experience. Low fat is better for me.
What is to be done about increased lactate?
In the short term I don’t know….One thing to be done would be fix the problem – stop the PDK overactivation and get the TCA cycle back to working more smoothly. Since Dr. Petterson, as do others, believes some sort of immune process is destabilizing energy production, ultimately the key would be to stop or inhibit the immune process. Reducing inflammation might be key although I imagine it would need to be targeted.
Reducing my own inflammation makes a big difference in my level of functioning. Conversely, an unrecognized infection that increases inflammation can trigger a serious flare that lands me back in bed. If I could only recognize that pattern when it first starts before the brain fog kicks in and drags it out…
Hi Cort, Could it also be an autoimmune response to our own energy metabolism?
If cyclofosfamide will help it looks like this. Very difficult matter energy metabolism. Respect and thanks for your good information. As always 🙂
That’s what I’m thinking. The immune system is attacking the energy metabolism in some way. It seems to be able to attack anything in the body! I’m wondering if the autonomic nervous system feeds into this as well. Something activates the stress response and destabilizes the energy metabolism.
When I was in college, I had a very serious case of mononucleosis that required a week in the hospital. I missed an entire term of school. I was an art major specializing in oil painting at the time. Oil paints have some very toxic ingredients. In 1980, our entire family had a horrible flu with upper respiratory and GI symptoms. Around this time we also had our house treated for fleas with Dursban, a pesticide that has since been removed from the residential market. My daughter and I have had ME/CFS since that time. Many people have no idea that they have been exposed to some very toxic substances in their home, work or schools. I am wondering if a virus combined with a toxin can result in long term illness like ME/CFS. Many of these toxins are stored in body fat so your body burden can last for years.
Interesting theory. Infection, Toxins. Immunesystem and nervoussystem. Connect the dots 🙂 Some kind of parasitic disease.
That’s intriguing about the Dursban. I’ve often wondered if exposure to a potent flea bomb (fogger) soon before I fell ill might have contributed. My husband and I were going away for a few days and I set a fogger in the bedroom before leaving—but then had to go back to get something while the fogger was still spraying. I don’t know what the contents of the fogger were, but given that Dursban (chlorpyrifos) is one of the few pesticides that fleas are susceptible to, I suspect it was in it. So, there’s that, and the tick bites (possible Lyme exposure), exposure to Bartonella from the cats & fleas, and a couple of incidences of whiplash affecting my cervical spine all around the same time. Are they all contributors?
I like the reporting on the science of what’s going on, but I’m still wanting to see treatments.
Believe me, I would love to be able to report on treatments that directly affect this energy production problem…..At least the research seems to, in general, to pretty consistently find problems with energy production and it seems to be more and more focused on the TCA cycle. Another presentation from the conference found that as well.
It certainly gives researchers a clue to look for autoimmune or inflammatory processes that affect the mitochondria. Hopefully, we’re narrowing things down.
For so many years I have said Autoimmune dysfunction and Inflammation…..pick your order.
I hope Dejurgen will put a bit more of what he and I have been studying as it ties in here. But I’m having a brain fog day. He is better on the technical and explaining. I can do the Summary and the research.
But there are supplements that can and will address this and I’ve been trailing them for about two months now. They do make a difference. I don’t have it perfected yet, however. But it addresses all that is written here.
There are also some diet tweaks that we recently tied in that we haven’t attempted yet. But, I feel IF we can have the willpower to do this diet…..it will make a huge difference. We will see. More later…….
Please review the attached paper published in TDM 9/2020 on transcriptomics of metaboloimmune abnormalities in CIRS/SEID. I looked for your email to forward a copy but finding none, I ask that you contact me. METABOLIC ACIDOSIS, PULMONARY HYPERTENSION, T REG DEFICIENCIES AND GREY MATTER NUCLEAR ATROPHY all employ proliferative physiology. FIXABLE!
Dr. SHOEMAKER,
I just wrote about you below and then paged up to read new post. I linked your paper in my post.
My Functional doc is Dr. Jennifer Smith. We have done the CIRS protocol with me and I’m one of the ones listed in your paper with Neuroquant findings. I’m sooooooo much better now. It was a blessing to find her. She and I have been a team and become friends. LOVE HER!
Waiting to do another Neuroquant and see what it shows now. I’m pretty certain it should be better!
Issie
Issie- Can you share those supplements and the diet plan. Would be of great help to us
Also Dr Shoemaker- Will be thankful if you share your paper with me. My email addr is anand.sundaram09@gmail.com
@Wayne: “Also, this recent study that reports “an alternative fuel source” being fatty acids is contradictory to the study recently in “Health Rising” that reported Amino acids were the alternative fuel source.”
This does not need to be a contradiction. It may be a rather good starting point to try and understand what is going on.
I envision two distinct “ME phases”:
* One where cells are at base / resting levels, have good enough access to oxygen and energy demands are low.
* Another one where we have too high energy demands for our poor cells and mitochondria to be able to deal with.
The first state would be one where our cells largely operate in aerobic mode. It would likely be a state with fairly low oxidative stress and sufficient oxygenation. Fat would be a good primary fuel in this state.
The second state would be one where our cells rely far more on anaerobic energy production. Fat is too slow for strong spikes in energy consumption. So then we need to look at either glucose / pyruvate and / or amino acids. I assume that in this state our low blood flows and poor oxygen release from the RBC make it a partly oxygen starved situation.
I assume this state to be high or very high on oxidative stress. If so, that would largly inhibit the conversion from citrate to D-isocitrate seen in the citric acid picture in this blog. If oxidative stress gets excessive, the enzymes for those steps are near entirely blocked.
These steps are however the first steps in the Krebbs (citric acid) cycle. They therefore would limit acetyl-coa usage and therefore pyruvate and fat usage by the Krebbs cycle. Many amino acids however can enter the Krebbs cycle after these by excessive oxidative stress near entirely blocked first steps.
As such, during such high (for us) spikes in energy, energy demand likely could be best met by using the glucose mainly for anaerobic glycolysis and use amino acids to bypass the blocked steps in the Krebbs cycle that pyruvate nor fat can easily bypass.
=> Summerized:
During rest, our cells may prefer to get most of their base energy consumption delivered by fat. It likely has some advantages towards reducing oxidative stress too. I’ll talk later about that.
During too strong activity, with too strong meaning the many times a day we exceed our peak capacity by near any exercise or thinking we atempt, fat is too slow a source and is replaced by glucose for glycolysis and amino acids for some more quick energy production. Some amino acids will be converted straight to fast glucose, others will enter the Krebbs cycle after the first steps of the Krebbs cycle that are near completely blocked when oxidative stress gets excessive.
There thus needs to be no contradiction here. Knowing in what state the studied cell is (at rest versus stressed too much) could be the key differentiator between what energy source is prioritized.
A textbook explanation! I – who never in the past differentiated between fatty acids and amino acids (were they the same thing I wondered?) – learned so much from this one comment. Thanks again, DeJurgen
?
You helped speeding up my learning curve soooo much when I was at worst and knew nothing about how the body worked. Glad to be able to return the favor.
Understanding that the Krebbs cycle is far more then a cycle where you insert things at the top and then it goes round and round is a big thing. It’s closer to an infite subway loop with many hop on hop off stations. Understanding that many chemicals in the body are “interconverted” back and forth rather then consumed goes a long way too. When combining both ideas, “magic” can happen.
Take this example:
Remember you wrote several times that when the body can’t produce enough ATP but needed it badly, it can convert two ADP to one ATP and one AMP. That one ATP can be used and that one AMP is lost as it can’t be converted back. That leads to phosphor loss and phosphor being low in us.
Well, let us use some magic here.
According to Wikipedia(Acetyl-coa_synthase):
“ATP + Acetate + CoA AMP + Pyrophosphate + Acetyl-CoA”
That arrow goes *both* directions so it could be used to convert AMP and phospor straight back to ATP. Now that would be something if producing Acetyl-CoA didn’t need to convert ATP to AMP to start with making the attempt pointless.
Here for we have Wikipedia(Pyruvate_decarboxylation):
“1 Pyruvate + 1 NAD+ + CoA → 1 Acetyl-CoA + NADH + CO2 + H+”
“Pyruvate decarboxylation or pyruvate oxidation, also known as the link reaction, is the conversion of pyruvate into acetyl-CoA by the enzyme complex pyruvate dehydrogenase complex.”
That is basically the top left step of converting pyruvate into Acetyl-CoA on the Krebbs cycle diagram in your blog.
Combine both and tada:
Preventing toxic pyruvate build up, alowing too high fat stores to be converted to energy and converting plenty of “irrecoverable” AMP that was produced during another crash into highly desired ATP all happening in a single go. It even produces some extra acetate, something I see quite good use for too (that however needs a bit more science checking to be done).
Even better? All could be done during this same resting phase: “rest to recharge” and build stocks for another exertion phase. Key to it? Don’t overdo it too long so that you still can recover all those valuable “waste” chemicals!
According to Wikipedia(Acetyl-coa_synthase):
“ATP + Acetate + CoA AMP + Pyrophosphate + Acetyl-CoA”
Had its two-way-arrow not copied, so it must be:
ATP + Acetate + CoA “insert-two-way-arrow-here” AMP + Pyrophosphate + Acetyl-CoA
A couple of interesting points in this to me, assuming I have understood it all correctly.
Firstly I was lucky enough to not be as severely affect by ME/CFS as I know many people are, and always wondered if this could be (in part at least) because I was very fit at the time of going down with it (flu was the trigger). Athletes develop through training a greater tolerance to lactate. Although it used to be thought of as entirely negative, it is now known that to an extent with training lactate can be recycled and used as a fuel. Although in the longer term it still slows the intensity of exercise, again it is delayed a little in a trained athlete.
Secondly the production of ketones and use of fatty acids as fuel. I have greatly improved since changing first to a low carb diet and going full keto. Which of course increases the use of fatty acids as fuel, rather than the carbs which the standard high carb, low fat diet conditions us to use as our primary fuel source.
For this idea of two different “ME phases” to work well, we would need plenty of amino acids and using them as fuel would create plenty of ammonia that is quite a burden on our bodies and livers. Clenring up too much lactic acid (produced by too much glycolysis) is also quite a burden to the liver. Both cleaning up excess ammonia and lactate consume plenty of energy too, something we are already starved of.
As we very often a day end up energy starved, this would be quite problematic. There once more seem to exist workarounds here.
Plenty of glycolysis is a good way to provide big spikes in energy to the cells, even during oxygen starved conditions. As said, that is however very costly. It produce plenty of pyruvate. In small quantities, pyruvate is a good anti oxidant and it protects the brain. In too high quantities, it becomes rather toxic. Then the body *urgently* needs to build it down.
The most known process to do that is to create lactic acid from pyruvate. That has the disadvantage to acidify our blood too much. The liver needs to convert lactate quickly back to glucose. That is a very costly process however, requiring 3 ATP to recycle it for every 1 ATP glycolysis delivered to the cells around the body using glycolysis to produce quick bursts of energy. Once again, if we are already low on energy… and oxygen as the liver *will* need plenty of oxygen for this recycling that is a costly business.
There are other workarounds however to decrease excessive levels of pyruvate. One is… constructing fat from it. The construction of fat during hypoxia has been described in several research reports to be protective against hypoxia. That includes fat building in the brain, in the cytosol (main compartment) of the cells and even in the mitochondria. Building up fat in the mitochondria apears to protect better against hypoxia then doing it in the cytosol as it requires less ATP to do so.
Notice that many inflammatory disseases lead to quick gains in weight. Notice also that many people with ME or other inflammatory diseasses easily get non alcoholic fatty liver diseasse.
Another way to reduce excess pyruvate during peak energy loads that is mainly used in the brain is converting glutamate and (excess) pyruvate to amino acid alanine and oxoaloacetate (something that can be used as fuel in the Krebbs cycle). The brain has quite a strong deposit of both glutamine (that can be converted to glutamate) and glutamate. This process again protects the brain against hypoxic damage.
Note: glutamate INSIDE the cells has not the same effect as the very excitatory glutamte in between the cells.
The downside of converting plenty of glutamate and pyruvate to alanine is a rise of alanine in the blood. That alanine has to be recycled to pyruvate and ammonia in the liver. That ammonia further has to be processed to urea in the urea cycle. That is a process costing even far more energy to the liver then recycling lactate.
However, if that is needed to save the brain from having permanent damage…
Hello dejurgen, I wanted to ask for your thoughts pertaining to my situation as far as I can tell. I have two SNPs that come up as “not set” — PDHA1 gene *(rs606231188) on chromosome X considered “probably pathogenic:” Pyruvate dehydrogenase E1-alpha deficiency.
Another one for “Pyruvate dehydrogenase complex deficiency” also “not set.” Gene: DLAT, rs2303436.
What do these mean in the context of your above formula explaining how to cycle ATP using pyruvate? I am just a layman trying to make sense of all this since a few years, reading this blog.
Basically: what should I take or do supplement-wise, what is missing, in order to get the pyruvate dehydrogenase deficiency working or at optimum levels?
Thanks.
Hi George,
To start: I do have NO MEDICAL SCHOOLING AT ALL. I’m autodidact since being ill with ME as conventional treatments did not help anywhere near needed.
=> THIS IS NOT MEDICAL ADVISE, only starting points for discussing with your real medical schooled doctor!
From a paper with title: “Thiamine-responsive pyruvate dehydrogenase deficiency in two patients caused by a point mutation (F205L and L216F) within the thiamine pyrophosphate binding region”:
” Thiamine treatment is very effective for some patients with PDHC deficiency.”
Thiamine is vitamin B1. Don’t go wild on supplementing. Talk to your doctor before starting it. I fail to remember now, but about all B vitamins seem to have pros and contras in ME. So for people without genetic pyruvate dehydrogenase deficiencies (it works not for all genetic PDH defficiencies) I personally wouldn’t megadose it.
As you have no way of knowing IF your genetic deficiency is B1 treatable, I’d consider starting more regular supplementation before potentially stepping it up. ALL AFTER TALKING WITH YOUR DOCTOR of coarse!
In some cases magnesium supplementing could help as it is another cofactor of PDH. See the paper with title “Pyruvate dehydrogenase phosphatase deficiency: a cause of congenital chronic lactic acidosis in infancy.”
“Addition of Ca++ and Mg++ to the inactivated enzyme caused a prompt return of the activity to normal in controls but not in the patient.”
Note: the patient appeared to have another genetic deficiency that blocked magnesium from helping.
Again ask doctors advise first. You also note that calcium can help. In western diets calcium deficiency often has more to do with a too acidifying diet rather then too few calcium intake. I read (have to check) that calcium and magnesium share some transporters and overloading with calcium can hamper magnesium uptake in the gut. In order to bypass poor gut absorpion, on can use magnesium sprays.
Again, ask doctors advise and go low and slow. There are downsides for magnesium supplementing too. Issie and I both had fire backs with several OTC supplements at doses labeled on the box while tiny doses did help us. The higher the dose, the greater the risk for side effects I believe. Remember that many of us have a chemical sensitivity and that seems for Issie and I to extent to common supposedly harmless supplements.
Another option is looking at what further inhibits PDH. One of them is pyruvate dehydrogenate kinase (PDK). Others are acetyl-Coa and NADH. It’s hard to influence acetyl-Coa with good effect. NADH can however stack during hypoxia. PDK is increased by HIF and HIF is increased by short term, intermittent and chronic hypoxia. Thus trying to prevent body wide and local hypoxia seems to be another option.
Trying to check if you have poor oxygenation during sleep due to things like sleep apnea or COPD (chronic obstructive pulmonary disease) can help. Following breathing theraphy *with a good licensed physical therapist* can help prevent periods of hypoxia too. Don’t try to learn it from a book or video. That backfired twice with me. Pacing helps to prevent a shortage of oxygen due to not having excess demand too.
Improving circulation by learning and doing regular light circulation exercises slowly helps improving blood flow. Again, consult a good professional physical therapist. Doing things wrong can cause more trouble then help you. Improved (not increased but better adapted!) circulation helps better distributing oxygen and removing lactate. By better removing lactate, part of the consequences of inhibited PDH are countered as inhibited PDH can create local and body wide lactate buildup. By better spreading lactate and bringing it back to the liver for recycling, peak local values can be reduced.
This is a starter, to be discussed with your doctor.
Now comes the clue of the above comments I made:
Pillaging amino acids for energy production very frequently as we may need would deplete our amino acids to the max and create a huge and very costly ammonia load to the liver. I know many of our amino acids are already low, but I guess that even wouldn’t suffice to satisfy our needs.
Creating plenty of fat from excesse pyruvate, getting it out of the cells, transporting it to adipose cells (specialized in fat storage), converting it back and forth to triglicerides to do so, releasing it from those stores again to use as fuel… would be quite a costly process itself. Not ever using that stored fat would lead to day-to-day increases in fat stored in our bodies till we become wider then we are tall…
So what are the workarounds here?
* When looking at Wikipedia(Alanine_transaminase), we see that not only glutamate plus pyruvate can be converted to alanine and alfa-ketoglutarate, but the process also can go the other way around. It doesn’t even cost ATP! That is a far cheaper way to reduce excess alanine then to convert it to pyruvate and ammonia in the liver. Even better, it replenishes our levels of amino acids to be used next time we get another burst in energy needs.
Well, actually it converts one amino acid to another one with more energy in it. That suffices for the stock of higher energy glutamate to be used in the brain again for use during another round of hypoxia and to help and save the brain.
Other processes can produce actual extra amounts of amino acids from pyruvate and ammonia. They are a bit more complicate to explain however.
The conversion of alanine back to glutamine seems to be a process idealy suited to be done once we get to rest again. Not overusing our brains and bodies too long is key to alow enough alanine to not to have to go throught the expensive recycling by the liver but rather be reconverted by the mitochondria to glutamine.
* As for fat, protecting the body from hypoxic damage seems to work best when the excess pyruvate is converted to fat in the mitochondria themselves. All that getting fat into the cytostol, then into the blood and into fat cells and back seems to be quite wastefull.
=> So, why not make the fat during hypoxia in the mitochondria themselves and keep it there?
That may seem impossible as the mitochondria aren’t known for fat storage. You can’t keep piling fat in them. So why not use that fat *build up in the mitochondria* to provide acetyl-coa to the mitochondria for energy production *once we return to rest*?
That will deplete the excess of fat in the mitochondria and create free room to produces and temporarily stock some more next time we hit too high energy demands or hypoxia again.
That tactics would have another advantage. Looking at Wikipedia(Fatty_acid_metabolism) we see that creating acetyl-coa from fatty acids creates NADH *and* FADH2 while creating acetyl-coa from pyruvate only creates NADH.
According to Wikipedia(Succinate_dehydrogenase), extra FADH2 should decrease the functioning of succinate dehydrogenase somewhat. As that is a Krebbs cycle enzyme, that may seem bad. But this enzyme reduces the speed of conversion of succinate to fumarate.
Too much fumarate is a very strong increaser of oxidative stress, so inhibiting succinate dehydrogenase a bit (by using fat as an energy source) seems to be able to reduce oxidative stress compared to using glucose / pyruvate. Having no excessive fumarate also should allow the urea cycle to work better and quicker, allowing to detoxify excess ammonia better too.
=> It seems that these “2 ME phases” are close to the equivalent of charging and discharging a battery:
During rest, extra energy and carbs are used to build and replenish high energy amino acids.
During rest, excess fat in the mitochondria are used to provide energy and create free room for more fat later. In doing so, they seem to reduce oxidative stress to lower levels.
During high energy loads, the “recharged” amino acids can provide for quick fuel for the Krebbs cycle. The not-so-much (during rest) used glucose can fuel quick glycolysis.
During high energy loads, there is enough room and low enough fat in the mitochondria for them to convert excess pyruvate (or better the acetyl-coa made from it) to fat and prevent hypoxic damage.
“Again glycolysis was fine but they found increased – not decreased – mitochondrial production in the muscles cells exposed to the ME/CFS serum.”
Maybe these healthy cells received the message “prepare for the next hit and clear your fat storages and use the energy to produce high energy amino acids” from ME patient serum?
Cort-I do not know how to send a suggestion on other topics so here goes…could you include a dietary/gut resource on the site?
The only plan I have found to heal the gut and be as inclusive as possible on foods over time is GAPS by Dr. Natasha Campbell-McBride, MD.
Thanks Jane. We do have a gut resources section but it’s hard to find as it’s on the Forum side of the website. I don’t think we’ve done anything on the GAPS diet though.
https://www.healthrising.org/forums/resources/categories/gut.141/
Cort, I have just been sent this link (to an article you wrote in 2016 in relation to the IACFS/ME Conference) from Solve M.E.
https://www.healthrising.org/blog/2016/11/17/iacfs-me-energy-exercise-metabolomics-chronic-fatigue-syndrome/
Cort! I need your help here. This article appeared in my FB flow. It is somewhat like the Norwegien article you have written about here. Is it along the same thesis, is it the opposite? At the moment my brain cells won’t work this out on their own. https://www.nature.com/articles/s41598-020-75406-w#Sec11
From the paper ‘Human Herpesvirus-6 Reactivation, Mitochondrial Fragmentation, and the Coordination of Antiviral and Metabolic Phenotypes in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome’ by Prusty’s and Naviaux’s teams:
“In this study, we studied quantitative changes in cellular as well as mitochondrial proteomics upon HHV-6A reactivation and found several significant alterations with close similarity to ME/CFS pathophysiology. PDP1 was down-regulated in response to HHV-6A reactivation. PDP1 helps in reverting the negative effects of pyruvate dehydrogenase kinases on pyruvate dehydrogenase. A decrease in PDP1 suggests a potential decrease in functions of pyruvate dehydrogenase, which is also reported in ME/CFS patients (28). We also found a decrease in the SOD2 level that can lead to high amount of ROS within the cell. We have previously shown that HHV-6 infection induces ROS in host cells and alters expression of glutathione reductase (29). These observations strongly support a pathological link between HHV-6 reactivation and ME/CFS.”
https://www.immunohorizons.org/content/4/4/201
Both Dr. Petterson’s study and a similar !Nature! (as in one of the very best scientific journals in the world) study linked by Kajsa pointed strongly to important PDH and PDK abnormalities. So, the question remained what it could point to?
My first guess went to hypoxia and Hif1-alfa and Hif2-alfa abnormalities. Those discuss how cells adapt to short term and chronic hypoxia. The science is very difficult however so I tried and find an easier explanation to start from.
This paper from “Thomas C. Vary & Stacy Hazen” titled “Sepsis alters pyruvate dehydrogenase kinase activity in skeletal muscle” says:
* “Chronic sepsis promotes a stable increase in pyruvate dehydrogenase kinase (PDHK) activity in skeletal muscle.” (it even used the same cell types as the Dr. Petterson study)
* “Sepsis was induced… …Five days later, mitochondria were isolated from skeletal muscle and PDHK measured in mitochondrial extracts. Sepsis caused an approximate 2-fold stimulation of PDHK.”
* “The activity of PDHK intrinsic to PDH complex was a significantly increased 3 fold during sepsis.”
=> *Chronic* sepsis does basically (near) exactly what was found in muscle cells by Dr. Petterson and the Tomas study published in Nature.
=> ME has been compared many times by researchers to be very closely resembling chronic sepsis.
=> Us having something resembling a chronic sepsis state could easily explain the strong PDH and PDK abnormalities Dr. Petterson and Tomas discovered and those abnormalities could explain much of our failure to produce enough energy during periods of increased energy demands.
Closely linked to chronic abnormalities in PDH and PDK enzymes is the easy rise seen in blood lactate. That doesn’t seem to happen in all studies or all patients however. There apears to be different ME subgroups.
Let us start from the following ideas:
* during any peak demand, fat metabolism can’t speed up enough to meet demand; such is also described in medical literature
* during any peak demand, consumption of glucose both anaerobically and aerobically is scaled up quite a lot in healthy people
* when PDH is crippled as the Petterson and Tomas study found, aerobically consumption of glucose barely can be scaled up. In simpler words: the mitochondria can’t use much more glucose at peak demand then at base demand in ME patients.
=> Other energy sources like anaerobic use of glucose or massive use of amino acids are needed to provide an ME patient with peak energy levels.
=> Even when massively using these other energy sources, ME patients can’t reach the same peak energy production levels as healthy people because healthy people *also* can use these extra energy sources on top of the mitochondria using a lot more glucose (which we can’t).
=> Both massive / frequent anaerobic use of glucose and massive / frequent use of amino acids as alternative fuel have been confirmed by studies in the form of quick lactate buildup and depletion of amino acid levels.
Why do I repeat all of this? Because massive / frequent anaerobic use of glucose (by the glycolysis pathway) *absolutely requires* that pyruvate quickly can be build down.
=> The enzyme lactate dehydrogenase is *key* to a quick build down of (at too high levels very toxic) pyruvate by converting pyruvate to lactate. When it succeeds, lactate levels will rise quickly. That is bad, but less bad then pyruvate levels rising too high.
=> When the enzyme lactate dehydrogenase is largely inibited, few quick enough options to decrease highly toxic excessive pyruvate exist. One of them is massively converting glutamate to alanine. That creates a massive ammonia load to the liver and it’s a process that is (even) a lot more expensive to the body then converting excess pyruvate to lactate. The other main option? Pull the emergency brake and crashing the body badly so it will lower energy use very fast and abrupt. In fact, excessively high pyruvate build up will also cause excessively high NADH build up. That will deplete NAD+ and without sufficient NAD+ mitochondria *can’t* continue working well. So they *will* fall flat on their face.
Lactate dehydrogenase is strongly inhibited by oxalates and urea.
See the paper with title “Urea and oxalate inhibition of the serum lactate dehydrogenase” from “Pauline M. Emerson and J. H. Wilkinson”:
“The serum lactate dehydrogenase (LDH) of 16 normal subjects was inhibited by 55 to 68% by the incorporation into the reaction mixture of 0·2 mM-oxalate.”
“The serum LDH of all 16 patients with liver disease was inhibited by 2 M-urea to a greater degree and that of eight out of nine patients with myocardial infarction to a lesser extent than that of the normal controls (45-62%).”
=> Having the combination of too high PDK, too low PDH *and* LDH inhibited strongly is *very* likely an *utterly crippling* combination for energy metabolism during increased demand.
=> According to the Petterson and Tomas study, ME patients already have too high PDK and too low PDH.
IF they would have too high oxalate or urea, then the situation could become crippling.
Urea is the byproduct of protein consumption. Trying to up energy production by having more amino acids to be turned to fuel is a double edged sword due to it. It can increase peak energy production as it is a good prime peak energy source. BUT it has very high chances to increase urea a lot, potentially crippling the key important lactate dehydrogenase enzyme.
That may be a reason why many “old school” naturapaths consider high protein diets to be highly inflammatory. Many of them were low on exact science but very good on observing patients. I value good observations. It may also be the reason why many healthy body builders (and some athletes) seem to be more prone to ME then one would expect: they very often combine excessive protein consumption with high peak or strong prolonged increased energy demands. If they don’t have a superb way to expell urea from their bodies that can become a tricky combination. They might be prepped for a single extra hit (like a strong infection) throwing them into ME.
Increased urea is also very common in dysfunctional gut situations like IBS. Why? A *large* part of urea is excreted in the gut by the gall blather adding it to the bile. The bile is then excreted in the gut and the many (good and less good) gut bacteria convert urea back to ammonia. That isn’t a bad thing. But when the gut hasn’t enough bacteria creating healthy SCFA, then that ammonia is largely *reabsorbed* and needs to be processed by the liver *again*. That requires a lot of work, energy and oxygen from the liver and it *will* increase blood urea levels. And those… …inhibit the all important lactate dehydrogenase enzyme.
Oxalates normally are dominantly produced inside the body itself. The remainder comes from high oxalate foods.
There are different mechanisms by which oxalates can increase inside the body. Many yeasts, fungi and bacteria are known to produce quite a high amount of oxalates. Part of them can be located in the gut, part of them can be located inside blood vessels, organs and even inside cells.
My invaluable research partner Issie has confirmed biopsys of biofilms full of pathogens been found by Dr. Fry in both her blood vessels and organs. She has microscope pictures of them. Pathogens like Borrelia are said to hide and live in cells. I don’t claim Borrelia is high on producing oxalate, but it is very common in those microbial life forms (as they use the glyoxylate cycle).
There are other ways to have excessive levels of oxalates. Using excessive levels of vitamin C is a tricky one here, as vitamin C has many very good properties. Under certain conditions however it can be converted into oxalates. In practice, there seem to be ME subgroups who do well on very high levels of vitamin C and subgroups where it keeps backfiring. It might be good to keep that in mind when you feel it keeps backfiring. Note: I didn’t said to not supplement vitamin C. I say I doubt that *mega* doses are good for all.
I also strongly suspect that frequent / recurrent hypoxia can increase oxalates too. There is good science pointing to it but I lack some data to confirm that idea so far.
As Issie and I experienced, food oxalates can make an impact on our health even if they only deliver a modest contribution to overall oxalate levels in the body. We are working on that idea too. Oxalates easily form very sharp crystal structures that are associated with inflammation too. Don’t start to try and reduce oxalate food drastically and quickly however, as that is known to fire back too. Things are more complicated then that. Preventing to eat only high oxalate foods on one day and none on another day may be a more reasonable start I *feel* (as in it not being solid science).
=> While reducing lactate dehydrogenase inhibition won’t be a fix all, it makes a decent chance to contribute to better health in a subgroup with people with ME. The good news? There seems to be several options to reduce lactate dehydrogenase (over)inhibtion. Issie and I are working on it. Please await our further ideas and observations as so very often doing things the “plain and easy” way in ME tend to backfire. It often requires quite a deep understanding of the underlying processes, good observation skills and plain luck.
There is what they call “oxalate dump”, if you lower oxalate too fast, your body responds in a not so good way. It is recommended to lower oxalate slowly to allow your body to slowly eliminate it. Then they (those who study issues in the body with oxylate) say to limit on a daily basis how much you consume on a DAILY basis. Combining too many oxalate foods with already the body possibly over producing due to yeast, molds, pathogens and we may have a HUGE problem. The amount said to be okay-ish is very low. But it would be hard to completely avoid it. And, NOTE……. don’t go too fast on this one. My dear, Dejurgen, didn’t listen to me on that one…..man, did he find out the hard way. So slow changes……IF you attempt this. (First he had way too many oxalate foods in one day and then the oxalate dump.)
As said earlier, we are new in this attempt and have much to tweak and learn as to how to make this work.
There are a few more things that can go along with oxylates (and other things) being an issue. Still in the research phase.
*** warning: very technical comment ***
When starting to look into the Petterson and Tomas research I suspected hypoxia to be involved. Quickly, it became clear that Hif1-alfa can increase PDK so it did fit the Petterson and Tomas research.
Note: hif stands for hypoxia inducible factor. It is a key molecule helping the body to prepare and cope better in low oxygen environments, preventing permanent damage or even death when oxygen levels hit too low by changing the gene expression of hundreds of genes to better cope with hypoxia.
There was a problem however, Hif1-alfa (the most studied hif sub-type) has a short-ish half-life time, quite a bit shorter then the 24 hours the cells were out of the body and incubated with their food source. That should have reduced hypoxia and have had Hif1-alfa to be largely disappeared. There are some chemicals that can stabilize Hif1-alfa. Stabilizing it gives it a far longer half-life time.
Hif2-alfa it appears is the molecule for chronic hypoxia addaptation. If hypoxia lasts roughly longer then half a day by what I did found, Hif1-alfa goes down to low levels again and hif2-alfa kicks in in force. Link A (that I will add in the next comment to try and bypass the WordPress spam filter) shows that. I did found another picture with that time scale being about a full day from left to right.
Unfortunately far less research has been done on hif2-alfa then hif1-alfa so far. So I can’t confirm if hif2-alfa also upregulates PDK. I did find preliminary information that increased Hif2-alfa also increases epithelial inflammatory pathways and decreases barrier integrity. So that isn’t good either. Link B (that I will add in the next comment to try and bypass the WordPress spam filter) shows that. There is even a hif3, but there is not much more known about it then that it exists it seems. It’s a fairly new domain. Some interesting things came up however:
Preliminary information seen in link C (that I will add in the next comment to try and bypass tha WordPress spam filter) shows that:
* hypoxia (and high fat diets) increases both M1 and M2 macrophages but increases M1 macrophages far more then M2 macrophages. That strongly increases the M1/M2 ratio.
* hypoxia makes M1 macrophages dominant and increases hif1-alfa iNOS and turns more arginine to NO synthesis.
=> While hif1-alfa is supposed to be short lived, it still seems to be associated in this picture with a chronic condition (obesitas). So there might be other factors prolonging the half-life time of hif1-alfa a whole lot beyond the short life time it sort of should have.
From Wikipedia(Macrophage_polarization):
“The imbalance of the macrophage types is related to a number of immunity-related diseases.[12][13] For example, it has been shown that increased M1/M2 ratio correlates with development of inflammatory bowel disease”
“M1-activation in vitro is evoked by treatment with TLR ligands such as bacterial lipopolysaccharide (LPS) ”
=> This (highly inflammatory) M1 dominant reaction can both be provoked by hypoxia and a strong bacterial infection. LPS is part of the shell of plenty of bacteria. When those bacteria are destroyed by the immune system, some LPS will be released and carried into the blood stream. When this type of bacteria form (very hard to remove) biofilms, they will shed their entire shell and hence release plenty of LPS into the blood stream. Remember that Issie had confirmed biofilms in both blood vessels and even in organs.
=> hypoxia and or bacterial infection problems (by means of increased LPS) are indirectly linked to IBS as both increase M1 dominance and M1 dominance is correlated with IBS.
The idea that NO production would be increased in ME patients is sort of weird, as we have too few NO for dillating our blood vessels. Buth when looking at Wikipedia(Nitric_oxide_synthase):
* “The inducible isoform, iNOS, involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism, as NO is a free radical with an unpaired electron. It is the proximate cause of septic shock and may function in autoimmune disease. ”
* “The inducible isoform iNOS produces large amounts of NO as a defense mechanism. It is synthesized by many cell types in response to cytokines and is an important factor in the response of the body to attack by parasites, bacterial infection, and tumor growth.”
* “NO produced by eNOS has been shown to be a vasodilator identical to the endothelium-derived relaxing factor produced in response to shear from increased blood flow in arteries.”
=> The NO produced by M1 dominant macrophages seems to be aimed “as bullets” specifically targetting pathogens. For blood vessel dillation we need eNOS to be upregulated, not iNOS as this M1 dominant state does.
A paper titled “Macrophage Endothelial Nitric-oxide Synthase Autoregulates Cellular Activation and Pro-inflammatory Protein Expression” says:
“Expression of inducible nitric-oxide (NO) synthase (iNOS) and “high-output” production of NO by macrophages mediates many cytotoxic actions of these immune cells.”
=> It seems that mainly the immune cells themselves produce more NO in this “M1 dominant state” “as a weapon against pathogens”. Many immune cells also release copious amounts of H2O2, HOCl… all in the very vicinity of twhere hose NO radicalls are released. Together they easily transform the released NO into even more toxic RNS (reactive nitrogen stress). That RNS is hugely reactive and toxic (to both humans and pathogens, but aimed at pathogens). The extra release of NO therefore does not seem to be in contradiction with reduced endothelial (vasodillating) NO in ME patients.
When using a search engine, there seems to be plenty information of the involvment of HIF in sepsis and immune regulation. So far, information goes all directions however. It does however start to create a picture of hypoxia, immune reaction (against pathogens) and PDH / PDK abnormalities to be strongly related.
Link A is figure 7 from a paper called “The Hypoxia-Associated Factor Switches Cells from HIF-1α- to HIF-2α-Dependent Signaling Promoting Stem Cell Characteristics, Aggressive Tumor Growth and Invasion” From “Mei Yee Koh, Robert Lemos Jr, Xiuping Liu and Garth Powis”
I still have to read it but need to rest urgently for not I’ll crash badly.
Link B is the second picture in the paper called “The role of hypoxia in intestinal inflammation” on researchgate.net called “Distinct-roles-of-HIF-1a-and-HIF-2a-in-IBD-Activation-of-HIF-1a-increases-barrier_fig1_292076359”
Link C is to be seen on diabetes/diabetesjournals.org in a blog titled “HIF-2α Blows Out the Flames of Adipose Tissue Macrophages to Keep Obesity in a Safe Zone”
I still have to read it but need to rest urgently for not I’ll crash badly.
I’ve been holding this one to tie in when it felt right. Based on Cort new blog and reading the paper from Shoemaker that seems to connect……this seems to fit now.
(Dr. Shoemaker is doctor who researches mold and biotoxin issues. He has named this CIRS and it stands for Chronic Inflammatory Response Syndrome. This can have strong genetic connections with getting and keeping it as a problem. I have those bad genetics and also did have mold exposure and biotoxin exposure. It is an ongoing, maybe lifetime, keep working on…..for me.)
Here is the paper of Dr. Shoemaker: (My Functional doc, Dr. Jennifer Smith in Arizona, is trained in treatment of this and is also aware of Dr. Shoemaker and Dr. Fry findings and treatment protocols.)
https://oatext.com/metabolism-molecular-hypometabolism-and-inflammation-complications-of-proliferative-physiology-include-metabolic-acidosis-pulmonary-hypertension.php#gsc.tab=0
Fungus/mold/pathogen issues and virus issues seems to fit here. And oxylates play in there with this. I think we may all have yeast/fungal/pathogen problems. I know they found that fungus in my blood and organs. Along with Lyme and co-infections and CIRS.
Dr. FRY really believes we need to be careful with magnesium and other minerals. Though he knows we are depleted of them. It feeds the pathogens and increases biofilm. But, there have been other doctors that feel we are sooooo low in magnesium and all the things that it does, we need to have it for other body functions. (I do know of one girl with this same fungus who kept taking magnesium and did the other things to break down yeast, fungus, pathogens and biofilm —– she got a whole lot better.)
He also is very much saying we need to be low fat and vegan. I did this for 3 1/2 years, faithfully. But started feeling really weak and I have felt better adding some small amount of meat back. But need to limit that. And going low fat is a better choice for me too. Wasn’t good and I didn’t do well with it. (Interesting what Dejurgen connected to issues with weight gain and some being a bit more fluffy than desired. Recent ideas on weight is that it is inflammation and autoimmune driven. Makes sense, as many with a weight problem does not seem to over eat, yet still have a hard time to lose.)
Here is the peer reviewed paper of Dr. FRY on connection with ME/CFS and these pathogens. (I do have microscopic pictures of it in my blood. And we did a thyroid biopsy and it was in a tumor that is there. This particular fungus has also been found in a brain tumor, prostate cancer and heart bypass plaque. It is also known to form tumors and build biofilm around itself.)
https://www.sciencedirect.com/science/article/pii/S2452231718300083?via%3Dihub
In the above study done with ME patients he found this fungus and other organisms with majority of them. Was a small study however. (NOTE…….pathogens?)
This below is the bioscienctist (Dr. Stephen Fry in Arizona) that found the fungus in my blood. This is an interview done of him. Seems he is still advising vegan diet and no sugar and is still using doxycycline and antifungal. He also RX Ivermectin to some, it is an antiparisite medicine. (NOTE: They are using Doxycycline and Ivermectin and Plaqunil as treatment also in COVID.)
https://kwikermedical.com/news/2019/3/23/is-heart-disease-cause-by-an-infection-fungus-and-why-a-plant-based-diet-garlic-and-edta-chelation-may-be-important-treatments
This was notes from Better Health Guy from a conference he attended and Dr. FRY spoke in April of this year. (I did not put all the notes he had to this interview, as there were so many. But only listed what would apply here.)
https://www.betterhealthguy.com/ehs2020
Stephen Fry, PhD spoke on “Neurologic Disorders – Multiple Sclerosis and ALS. The Potential Roles of Fungi and Mercury” and shared:
He started with CFS patients which were similar to MS patients.
Next Generation DNA Sequencing is like a “tricorder” of infectious diseases.
Fungi: 300 clinically relevant; 2017 estimate suggests 2.2 to 3.8 million species of fungi.
Infections happen because the immune system is weak.
Initial hit to the immune system could be viral.
Numerous viruses with very few tests for them.
Can have a viremia that settles down into the tissue and leads to autoimmune disease.
An increase in Funneliformis mosseae is seen in MS patients.
Fungal toxins may be the underlying cause of MS.
HLA DRB1 15 a genetic risk for MS and associated with fungal infections.
Many biomarkers of MS are consistent with fungal infections.
In 2008 study, serum Candida antibodies were found in 7 of 8 with MS.
CFS/ME has many similarities with MS; lesions as a prelude to MS; evidence of fungi, positive urinary mycotoxins.
Normal control blood shows some fungi but in unhealthy people, the fungi get a foothold after some kind of damage.
Most antifungals have liver toxicity or kidney toxicity; need to be used for a long period of time.
In vascular obstructive disease, plaques are full of fungi, protozoans, and algae.
CCSVI has lost favor, but vegetations are observed by ultrasound. Mostly eukaryotes.
Environmental fungi are tough fungi; not easy to address with antifungals.
Whole food, plant based diet often helpful for MS.
Neurodegenerative disease may have a first hit being viral and then fungi may then start to colonize and lead to disease.
Metals may exacerbate the condition.
Treatment: diet, exercise, chelating agents, herbals, ozone, C, HBOT, exosomes, angioplasty, biofilm busters, RX antifungals; new drugs are needed.
MS and ALS could have fungal origin with mercury as a co-factor.
Antifungals have shown efficacy in MS.
Immunosenescence may be related to viruses. RNA viruses like Chikungunya and alphaviruses lead to a viremia; disappear from the bloodstream in a month or two but moves into the tissue. Many more viruses than bacteria.
Over time, the viruses wear down the immune system. It is not age, but viral load.
Patients likely have a fungal issue and a viral issue.
It may be that the fungi serve a role in nature creating mycotoxins that may be suppressing the viral issue which is the core issue.
___________
>>>>>There possibly being pathogens, fungus, yeast in our bodies and it causing inflammation and forming oxylates. Makes sense that lowering these organisms and lowering oxylates may be a BIG HELP to us all.
(And note that Dr. FRY feels virus plays a part in the illnesses too. I’m addressing that and also Post Lyme Syndrome with the same herb. LOMATIUM)
Experiment in progress ……..Dejurgen and I are working on it.
And also hypoxia and issues with lack of oxygen seems to be a big issue too. I’ve got a trick for that one too. Not only breathing exercises but retraining mouth and tongue position and using mouth tape to sleep. It helps you desensitize to more carbon dioxide and also increases oxygen. I actually no longer need a CPAP and have/had both central and obstructive apena. My oxygen levels staying up at night and during day pretty well now.
Hi Dejurgen – I post here instead of nesting, because I think it may get lost that way.
+ For ALT to work, it needs Pyridoxal Phosphate (PLP, a B6 vitaminer).
B6 deficiencies will then affect the reversible conversion of alanine + 2-oxoglutarate to pyruvate + glutamine.
And perhaps how energy demands are met and excess pyruvate dealt with.
I’ve been looking over my lab work. There was a sharp decline in ALT after 2008.
AST levels also went downhill a bit, but not that drastic.
It would be a good idea to get the three tested at the same time, B6 ad ALT/AST, from now on…
There’s an important bit also on the ratio of ALT/AST (because levels outside the norm often elude our labwork, and doctors tend to dismiss us as being OK, I’ve gotten used to studying the relationship of substances tested, which is how I got to looking into B6):
https://www.labpedia.net/sgpt-alt-alanine-aminotransferase-serum-glutamic-pyruvic-transaminase/
++ I have high plasma B6 (PLP) level, but symptoms and labwork (such as ALT and complete blood count) indicate deficiency. This is an issue some of us with weird diseases have. How many? we don’t know because it is not regularly tested. ++
+ In this lecture (I’m assuming the sources are legit) one can begin to appreciate the relationship between B6 – ALT – AST – ALP -Taurine – Sulfurs and the 1-carbon metabolism.
https://www.slideshare.net/openmichigan/020112r-van-dykelivertests
+ Low PLP also affects oxalates, in that it is a cofactor of AGXT. “A defect in AGXT is what causes primary hyperoxaluria, causing a lifetime buildup of oxalate generated mainly in the liver, and in that condition, the quantity of oxalate that accumulates can be fatal.” Here I am quoting Susan Owens, the biochemist behind the low oxalates group. So a B6 deficiency may lead to a higher accumulation of oxalates in the body.
+ Pyrodoxamine (another B6 vitaminer) has a function in “Maillard reaction and can block the formation of advanced glycation endproducts” Is this relevant to us?
https://en.wikipedia.org/wiki/Pyridoxamine
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
+ Thiamine pyrophosphate (TPP), which is derived from thiamine (vitamin B1) is a Pyruvate Dehydrogenase coenzyme:
“It is associated with the E1 subunit of PDH. In cooperation with an aspartate residue in the active site, TPP forms a carbanion, that is, a negative charge on a carbon atom. The TPP carbanion is resonance-stabilized; an electron can move back and forth between the carbanion and the neighboring cationic nitrogen. Carbanions are very powerful nucleophiles, and the TPP carbanion functions as such in the decarboxylation of pyruvate.”
http://watcut.uwaterloo.ca/webnotes/Metabolism/TCAcycle.html
(This is a really nice resource on metabolism 101 for those interested)
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
This may then indicate two sub-groups within ME/CFS: how a B6-deficient metabolism adapts for the glitch in energy production and how a B1-deficient one adapts.
Cort: you mentioned that “the energy production problems in ME/CFS are not homogeneous, though: another study suggested that several metabolic subgroups exist.”
Has this study been shared yet – do you know who did it? – or was it only mentioned in the presentation?
+ I encourage having B6 and B1 plasma status assessed. Even with normal levels, proceed with extreme caution. There are people who end up with extreme symptoms due to supplementation that are not easy to walk back. Make informed and savvy decisions +
Of course, there may be other sub-groups. These are the ones in my radar – the B6 and the B1 deficient ones.
I found B6 or P5P to give me narcolepsy light. With unrestful sleep and vivid dreams. And frequent waking.
B1 however, is one of my must haves. Has really helped my energy, muscle strength and endurance.
This underscores how different we all are:
B1 is a bit screwy for me… 🙂
I can’t tolerate B6 either I read an article about a defect in breaking it down I can’t recall just now how much looking I did into that one or if I know that I have it or not. I just know I can’t take B6 B1 is ok and when I have brain fog B1 is top of the list. At this moment I am working my way out again of a bout of jerking and ALS like movements… my GPs term not mine. As for my mind it is like the blackboard has been wiped but I get snippets of things… I think I got to the can’t break down B6 from my genetics info. but can’t be sure just now. I did more work on that one and ended up with Pantethine as a replacement for B6 .. again like the kid at the black board who can’t show her work.
I had surgery in Nov and was told to stop all supplements and I remained mostly ok for a time… I was allowed to keep magnesium
I after a complete face plant in the MCS .. my last dx had chronic fatigue fibro dx for 30+ years. I have gone back to supplements… slowly not clear on what to take for what symptoms. I have a staple of a few mag citrate B2 Vit E and C Selenium… B1 I take in moderation. Today after reading this I added the pentathine just moments ago.
I am not suggesting anyone else should do anything this is just my experience. I have found green juicing helpful when I am up to making it and gather in all the organic things I need to make it.
I can say pantethine has been helpful for me in moderation. As for the B2 I find natural factors the best for pantethine nothing but AOR… just what my body seems to be happiest with.
When I was having an issue with what I thought was hi ammonia I did some research on probiotics from that I found and used the baby version of BioGaia… which seemed to help. The particular strain of L. reuteri DSM 17938 when I was digging showed to be a ammonia lowering sort…. I am not saying to take anything… this is just what I found helpful. Lord knows many things I have tried that worked for others did not help me. Just if you think it may help look it up do your own research start low and slow.
Out of advice for the moment. I am toying with trying DHEA… we will see.
PS this is in response to Issie who had the same bad time with p5p and B6 as I did…
I just read a very provocative article that emerging research is showing that some people who have had Covid are acquiring RA or Lupus like conditions post infection.
https://www.nytimes.com/2020/10/27/health/covid-antibodies-autoimmunity.html
The article also mentions that blood flow problems are also part of the mix. Perhaps Covid triggers the same kind of mysterious plasma factor that researchers are finding in people with ME/CFS.
Humm…perhaps some of the medications for autoimmune conditions like these might help us. Steroids anyone? Pulsed micro-doses?
I know that steroids in general are bad news, but maybe this is another clue in our search for help for ME/CFS.
Fascinating – thanks Nancy. Steroids can be quite helpful for a subset of ME/CFS patients.
Two very interesting studies, thank you for presenting this so clearly. With the mitochondrial dysfunction I wonder how much is specific to CFS… After all, some degree of mitochondrial dysfunction seems to accompany many other diseases, and who knows if all this is not just a reflection of the crappy perfusion.
The work of Lenny Jason is thrilling. What a gold mine. He could in sequential blood samples find out at what time point possible autoantibodies pop up – be it GPCR antibodies or CNS neuro-autoantibodies which have recently come into focus at least with regards to GWI: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7563126/
Hope many CFS researchers team up to harvest the secrets of this unique cohort!
Is there any explanation for elevated cholesterol with these studies?
Even with the most expensive meds (injections) my very elevated numbers don’t go down and my cardiologist is fishing in the dark.
Hi Marlene – I hope you get to read this:
Caroline Elizabeth has ME/CFS with very high levels of cholesterol.
She has superb analytical and writing skills, and tries to go over the science behind what she is doing with her approaches.
She has a series of articles starting here:
https://chronicallycaroline.com/2019/06/02/keto-and-me-part-i-an-elevated-heart-rate/
And one where she goes over what her doctors are recommending as far as medicine to deal with the high cholesterol – taking ME/CFS dysfunction in consideration – it’s pretty impressive.
Good luck!
Five years late to the party but will post anyway. My lactate is elevated and i no doubt am in glycolosis much of the time now. I likely have been using AAs as a fuel for a while, given the high ammonia level in urine despite low protein intake and low levels of AAs in urine except for a few oddities. I appear to be catabolizing muscle for protein and perhaps thats why urine beta alanine is high, which in turn lead to taurine wasting in urine with a documented taurine deficiency in blood. Taurine supplementation oy helped for a few months, after which its just backfiring and now stripping my blood of cysteine which is getting dumped overboard in urine. It also may have been the disrupior of my being able to use taurine to conjugate with bile acids. I would like to come back to that if i don’t run out of steam. Other anomolies in urine are highidh seine and proline , both of which are low now in blood.
But heres the kicker or one anyway. I am now no longer able to oxidize fatty acids. Besides symptons of gi fat intolerance, recent metablomics test shows i am accumulating intermediaries in the process of attempting and failing to oxidize fatty acids. Its most obvious for long chsin fatty acids bith unsaturated snd saturated but only because i cant i take much if sny of others. On top of that, i have too little conjugates of bike acids to taurine and glycine, especially taurine. I have ample glycine in blood (unlike taurine) yet its not using it to conjugate much. Stool tests find increased cholesterol yet no elevatiobn of cholesterol in blood (but will check again) Mast cell issues do not help with its constraints on what’s tolerated (rg allergic to coconut and milk so there goes mcts). Fast forward a bit, i cannot stop losing weight.everything was worsened by a food-bourne infection (say goodbye to the short chsin fatty acids too!) but the weight loss there before. I am basically starving to death and not sure how many more months until the inefficient catabolism fails to keep up
Energy wise it is fascinating. In an earlier stage, i had profound fatigue and it was the limiting factor to hsvibg a life. Now i long for those days. I am i stead in constant sympathetic nervous system activation (and dysautonomia) perhaps my bodys last attempt to function.