

Longtime readers of the Health Rising blog may remember an article that Cort wrote in 2013 about the promising results of a small study that found marked reductions in fatigue among three individuals with Fibromyalgia who took high doses of thiamine (Vitamin B-1).
In the intervening years, several important studies on high-dose thiamine have been published, including a well-designed randomized controlled trial in November 2020. The publication of this landmark study provides a good opportunity to re-examine the evidence on high-dose thiamine and consider whether this supplement might be helpful for some people with ME-CFS, Fibromyalgia and Ehlers-Danlos Syndrome.
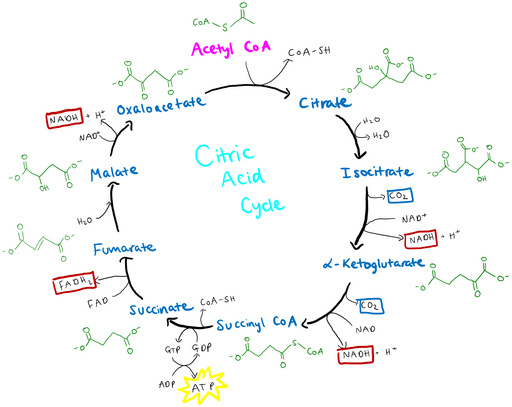
Thiamine, also known as Vitamin B-1, is an essential nutrient that plays a critical role in aerobic cellular respiration. A thiamine derivative, thiamine pyrophosphate, is necessary for the citric acid cycle to function properly and produce an adequate amount of the ATP molecules that the body uses for energy. Thiamine deficiency is a serious health problem, but most people in the western world get all the thiamine they need through a healthy diet.
The recommended daily allowance of thiamine is 1.1-1.2 mg/day. By contrast, the 2013 study on fibromyalgia used 600 to 1,800 mg of thiamine daily, more than 500 times this amount. This is the first clue that the study may involve something other than simple supplementation to remedy a vitamin deficiency.
The fibromyalgia study was one of a series of case studies published by the Italian physician, Antonio Costantini, and colleagues in 2013-2018, finding reductions in fatigue from high-dose thiamine among individuals with a range or neurological and inflammatory conditions, including Inflammatory Bowel Disease (IBD), Multiple Sclerosis, Parkinson’s Disease, and chronic cluster headaches.
These studies were all fairly small, and none compared the results against a control group of individuals who did not receive the treatment. Without the rigor of a randomized controlled trial, it was impossible to know for sure if the benefits were due to thiamine, the placebo effect, or some other explanation.
With the publication in November 2020 of a randomized controlled trial of high-dose thiamine by Palle Bager and colleagues at the Aarhus University Hospital in Denmark, that criticism has largely been addressed. This randomized trial confirmed the results of one of the earlier Italian studies, finding significant reductions in self-reported fatigue from high-dose oral thiamine hydrochloride over a four-week period among patients with quiescent (i.e., non-active) IBD and long-term fatigue.
In this guest post for Health Rising, I describe the results of this new study, explore potential explanations for why high-dose thiamine might relieve fatigue, and describe why I think some people with ME/CFS, Fibromyalgia, and the neurological complications of Ehlers-Danlos Syndrome (EDS) might benefit from it.
I conclude by asking those of you who have tried high-dose thiamine to complete a survey to help the field better understand whether and for whom high-dose thiamine might be helpful. If the survey results suggest that at least some people with ME-CFS, Fibromyalgia or EDS might benefit, I hope to use the results to encourage researchers to conduct a more rigorous study of its potential benefits for individuals with these conditions.
Study Results

The randomized control study found a significant decrease in fatigue in those taking the high-dose B-1.
Between November 2018 and October 2019, Palle Bager and colleagues in Aarhus, Denmark enrolled 40 individuals (35 female, 5 male) with quiescent IBD and fatigue lasting six months or more in a randomized, double-blind placebo-controlled crossover trial.
Half of the individuals were randomly assigned to received high-dose thiamine for four weeks, followed by a four-week washout period and then four weeks of placebo. The other half got the placebo first, then the washout, and then high-dose thiamine. Neither the patients nor the researchers knew who was in which group.
The study used 300 mg tablets of oral thiamine hydrochloride, assigning a daily dose by gender and body weight (BW) as follows:
- “Females: BW <60 kg: 600 mg (2 tablets), BW 60-70 kg: 900 mg (3 tablets), BW 71-80 kg: 1200 mg (4 tablets), and BW >80 kg: 1500 mg (5 tablets);
- Males: BW <60 kg: 900 mg (3 tablets), BW 60-70 kg: 1200 mg (4 tablets), BW 71-80 kg: 1500 mg (5 tablets) and BW >80 kg: 1800 mg (6 tablets).”
It is not clear whether the study participants took their pills all at once each day or spread out into divided doses, but they were advised not to take the pills in the evening due to the risk of temporary sleeplessness.
The primary outcome consisted of a measurement based on Section I of the IBD-F fatigue scale. Section I of this validated scale consists of five questions to the patient asking about fatigue over the past two weeks. To be included in the study, participants needed to have a fatigue score greater than 12, which is about 70 percent higher than the mean fatigue score of 7 in the general population. The researchers defined a reduction of 3 points or more as clinically relevant.
They found the patients’ reported fatigue declined by an average of 4.5 points while taking high-dose thiamine as compared with an increase of an average of 0.75 points while taking the placebo, a statistically significant difference. The results, after excluding three patients who had a flare-up of IBD or needed an iron infusion during the study, found a net reduction of 4.7 points in the average fatigue scale score attributable to high-dose thiamine.
There was no significant difference in results among those with and without a thiamine deficiency at the time they enrolled in the study. The finding that reductions in fatigue were not limited to those with a thiamine deficiency accords with the results reported by Costantini and colleagues across a range of conditions.
Why might thiamine reduce fatigue?
If high-dose thiamine does not work by addressing a thiamine deficiency, through what mechanism does it reduce fatigue? The short answer is we do not know for sure, but several hypotheses have been offered.
Building on a hypothesis first advanced by Costantini, Bager and colleagues suggest the possibility that high-dose thiamine may have helped compensate for a defect in the active transport mechanism through which thiamine is normally absorbed. “While the effect of high-dose oral thiamine was highly significant in our study,” the authors write, “its exact mechanisms still need to be explored and investigated. The theory of a dysfunction in thiamine transport from blood to mitochondria remains a plausible explanation.
The participants in our study were exposed to high doses of thiamine which induces passive diffusion that will add thiamine to the cells and the mitochondria. Consequently, the carbohydrate metabolism can normalize, and a reduction of fatigue is likely to follow.”
In a recent comment on a blog post, noted thiamine expert Derrick Lonsdale offered an alternative hypothesis to explain what he terms as the “miraculous” clinical effects of high-dose thiamine.
“[T]hiamine, and particularly its derivatives, are being used as ‘drugs’. It is nothing to do with simple vitamin replacement. The enzymes that require thiamine have been deprived of it for so long that it can be expected that they have deteriorated ‘physically’ in their metabolic responsibility. The cofactor has to be used in megadoses in order to stimulate the enzymes back into their normal function.”
As Lonsdale acknowledged, this hypothesis still needs to be evaluated through research.
In a letter to Alimentary Pharmacology and Therapeutics, the medical journal that published Bager’s study (the authors’ reply is here), I offered a different hypothesis that builds on two other recent studies of high-dose thiamine.
In 2013, Özdemir and colleagues found that high-dose thiamine inhibited three carbonic anhydrase isoenzymes nearly as well as acetazolamide (Diamox). Inn 2021, Vatsalysa and colleagues found that high-dose thiamine tamps down the pro-inflammatory Th-17 pathway believed to play a role in the COVID-19 cytokine storm.
Building on these findings, I propose that the benefits of high-dose thiamine in relieving fatigue and generating other symptomatic improvement in patients with a diverse range of neurological and inflammatory conditions may be due to thiamine’s role as a carbonic anhydrase inhibitor.
I hypothesize that the benefits accrue through one or more of four potential pathways:
- by reducing intracranial hypertension and/or ventral brainstem compression;
- by increasing blood flow to the brain;
- by facilitating aerobic cellular respiration and lactate clearance through the Bohr effect; or by
- tamping down the pro-inflammatory Th-17 pathway, again through the Bohr effect, possibly mediated by reductions in hypoxia-inducible factor 1.
This is a lot to unpack, so let me offer this high-level overview, and refer readers interested in the technical details to a blog post I wrote on Medium that explains my theory in more detail, with full citations.

Could high-dose thiamine help with blood flows to the brain, intracranial hypertension and aerobic functioning?
In a nutshell, I am proposing that taking high-dose thiamine is a lot like taking Diamox (the brand name for acetazolamide – the most common treatment for intracranial hypertension, but without many of the worrisome side effects. Rather than working by addressing a vitamin deficiency, high-dose thiamine operates by reducing intracranial hypertension (and possibly ventral brainstem compression as well) through a reduction in cerebral spinal fluid.
In the process, by inhibiting carbonic anhydrase isoenzymes, high-dose thiamine produces carbon dioxide, which leads to increased blood flow to the brain and an increase in the availability of oxygen at the cellular and tissue levels for aerobic respiration, reducing reliance on anaerobic respiration and helping to clear lactate. By reducing hypoxic conditions, the increased oxygenation also reduces the levels of a mediator (hypoxia-inducible factor 1) that triggers the pro-inflammatory Th-17 process, helping to counter inflammation.
I will be the first to admit that these ideas need to be carefully evaluated through rigorous research. My goal in proposing these hypotheses is not to offer them as gospel but rather to stimulate research into the mechanisms through which high-dose thiamine operates. These mechanisms are important both for predicting the potential conditions that high-dose thiamine could help treat and for clarifying the limitations and cautions that should be applied to its use.
The hypothesized mechanisms I describe above are alternatives in the sense that one or more may be accurate while the others may be inaccurate. It also may be the case that some individuals benefit through one mechanism while others benefit through another mechanism, which may help explain why people with a wide range of conditions report benefits from high-dose thiamine. Some individuals may even benefit simultaneously through multiple mechanisms.
Could high-dose thiamine help people with ME/CFS, Fibromyalgia, or EDS?
More research is needed to answer this question definitively, but I believe we know enough to suspect this research would be worth conducting. The 2013 study by Costantini and colleagues finding benefits among people with fibromyalgia provides a basis for conducting a larger and more rigorous study of the potential applications of high-dose thiamine for people with fibromyalgia.
Similarly, the case reports and discussion included in the Driscoll Theory provide a basis for follow-up research on the potential benefits of a carbonic anhydrase inhibitor like high-dose thiamine for people with certain neurological complications of EDS. Driscoll’s book argues that people with some neurological complications of EDS can benefit from acetazolamide (Diamox). She attributes the results to reductions in intracranial hypertension, a known complication of EDS. I wonder, as well, whether a carbonic anhydrase inhibitor could help with ventral brainstem compression caused by Craniocervical instability and Chiari malformation.
The case for predicting the benefits for people with ME/CFS is more circumstantial. A 2020 study found signs of possible intracranial hypertension in 83% of 205 individuals with ME/CFS that had an MRI performed. The reductions in cerebral spinal fluid achieved through a carbonic anhydrase inhibitor like high-dose thiamine might potentially be helpful for individuals with these and other neurological conditions.
There is reason to believe that some of the other pathways may also apply. For example, many individuals with ME/CFS have reduced blood flow to the brain. As Cort has summarized in past blog posts, this might be related to the finding in several studies of reduced levels of CO2 in people with ME/CFS. The authors of these studies hypothesize that these reduced CO2 levels may be narrowing blood vessels, constricting the flow of blood to the brain. By producing CO2, high-dose thiamine could potentially remedy this problem and improve blood flow to the brain.
The Gist
- A randomized-controlled trial of high-dose thiamine found that it reduced fatigue in people with quiescent IBD.
- The outcomes did not differ for individuals with or without a thiamine deficiency at the start of the study.
- The exact mechanism for thiamine’s effects on fatigue is not clear. The author of this post hypothesizes that high-dose thiamine’s effects might be due to its role as a carbonic anhydrase inhibitor, which could reduce intracranial hypertension and produce CO2 that increases blood flow to the brain, tamps down the pro-inflammatory Th-17 process, increases aerobic respiration and clears lactate.
- There are reasons to believe high-dose thiamine could potentially help people with ME/CFS, Fibromyalgia and the neurological complications of Ehlers-Danlos Syndrome. Rigorous research is needed to assess whether this might be the case, and if so, who is most likely to benefit.
- Carbonic anhydrase inhibitors are powerful medications that have the potential to interact with a number of other medications and supplements.
While a preference for anaerobic respiration is apparently common among people with ME/CFS, this is often attributed to mitochondrial issues, and I am not quite sure how a carbonic anhydrase inhibitor could help with this. (The passive transport theory articulated by Bager and Costantini might potentially be useful, however.) On the other hand, the production of carbon dioxide through carbonic anhydrase inhibition could potentially help to improve lactate clearance, which Vink identifies as a problem in ME/CFS.
Hopefully, future studies of the potential benefits of high-dose thiamine for people with ME/CFS, fibromyalgia and the neurological complications of EDS will go beyond studying the general concept of “fatigue” to assess a range of more specific outcomes. Potential benefits could include improvements in mental acuity / brain fog and certain kinds of headaches – due to reductions in intracranial hypertension – and reductions in post-exertional malaise due to increases in aerobic respiration and lactate clearance.
While it is just one case, my daughter, who has EDS, craniocervical instability and chiari malformation, appears to have experienced improvements in brain fog and post-exertional malaise from high-dose thiamine (though at particularly high doses of thiamine, she actually reports an increase in general tiredness).
To help bolster the case for conducting a rigorous study of whether high-dose thiamine benefits individuals with ME/CFS, fibromyalgia, or the neurological complications of EDS, it would be helpful to learn more about whether individuals with these conditions have benefitted from high-dose thiamine. Please don’t start high-dose thiamine just to participate in this survey, but if you have already tried high-dose thiamine (which I define as a daily dose of 200 mg or more or oral thiamine), and feel comfortable completing this survey, please go ahead and do so.
Please complete the survey whether your experience has been positive or negative. I will summarize and report out the results in a future column.
Cautions and Limitations
If I am right that high-dose thiamine is a carbonic anhydrase inhibitor with a potency approaching acetazolamide, there are many cautions that should apply to its use. These include the risk of potassium deficiency (particularly if combined with a diuretic, including herbal diuretics) and the potential to form kidney stones. The combined use of high doses of aspirin and acetazolamide (another carbonic anhydrase inhibitor) has been reported to lead to salicylate toxicity. And people with intracranial HYPOtension would likely feel worse from high-dose thiamine, even as people with intracranial HYPERtension potentially feel better.
Driscoll reports a phenomenon in which acetazolamide sometimes stops working after a period of time. This phenomenon is also reported in the older literature on acetazolamide, which also describes a general malaise that appears to be related to mild acidosis. The older literature reported the problem resolved with sodium or potassium bicarbonate, though acetazolamide today comes with a warning against the routine co-administration with sodium bicarbonate due to the risk of kidney stones. In addition to taking sodium bicarbonate, Driscoll also recommends lowering the dose.
Finally, there is some suggestion that thiamine may be a histamine liberator and DAO inhibitor (though in theory, reductions in intracranial hypertension and brainstem compression could potentially lead to improvements in MCAS symptoms). My daughter seems to have experienced some shifts in food tolerance after starting high-dose thiamine, but no marked improvements or worsening.
This all suggests the importance of clarifying whether high-dose thiamine really is a carbonic anhydrase inhibitor as predicted by Özdemir’s 2013 in vitro study, and providing clear guidance to practitioners and patients on how to avoid or reduce complications.
For more discussion on the potential of high-dose thiamine to help people with ME-CFS and the neurological implications of EDS, including full citations, see my Medium post on this topic.

Jeffery is a father of a daughter with ME/CFS and allied disorders.
About the Author
I am the parent of a teenage daughter who has been diagnosed with Ehlers-Danlos Syndrome, hypermobility type; Postural Orthostatic Tachycardia Syndrome; Mast Cell Activation Syndrome; and Chronic Fatigue.
I have prepared this post to stimulate further research, and not to provide medical advice. I am not a medical professional and do not have medical training.






@Jeffrey Lubell: Thanks so much for your great overview of the effect of high dosis B1 supplementation on FM/ME/… diseasses.
Issie and I have been discussing her improvement with high dose B1 more then once. I am weary to use anything dosed so much higher then daily recommended doses or needs as it has the potential to crowbar key biological processes. Yet, I cannot deny many patients are reporting significant differences when taking those high doses.
Based on what you wrote and some quick search, I have two things I consider making a chance to be rather important here.
One you reported was:
“In 2013, Özdemir and colleagues found that high-dose thiamine inhibited three carbonic anhydrase isoenzymes nearly as well as acetazolamide (Diamox).”
=> Would by chance people who start to take high dose B1 within a few days feel a really remarked and even outspoken ability to breathe, to get oxygen from breathing and feel an ease of breathing they didn’t had in a long time, to need to hyperventilate so much less then before?
If possible, I’d like to see as much answers yes or no or some relevant comments to this question. The answer to it may be rather important to further understand what is at work with both high doses of B1 and even ME/FM diseasses in general I believe.
So: thanks in advance for all willing to reply. Don’t hold back to report either yes or no answers, we need not only successes to be reported.
Thanks for the note and your question. I am glad to hear Issie is benefitting from high-dose thiamine! The short answer is that what you describe may well be possible, but more research would be needed to confirm. Much has been written about the use of acetazolamide as a respiratory stimulant, particularly for individuals with COPD and in connection with Acute Mountain Sickness. To the extent thiamine is a carbonic anhydrase inhibitor similar to acetazolamide, it may well help in these circumstances as well. However, there are debates about the precise mechanisms through which acetazolamide operates and some believe that carbonic anhydrase inhibition is only one of the mechanisms, so it’s possible thiamine may not do everything that acetazolamide does. The field would very much benefit from a study of the potential of high-dose thiamine to serve as an alternative carbonic anhydrase inhibitor. In the meantime, I am trying to collect information on peoples’ experiences with high-dose thiamine, so if this has been one of the effects, it would be helpful to record this in the survey. Thanks again for your interest.
It’s pretty clear when carbonic anhydrase is being inhibited…carbonated beverages all taste flat. Diamox produces this effect strongly. Topiramate, a milder carbonic anhydrase inhibitor, also creates a milder form of this altered taste sensation. However, I get none of this from high dose thiamine, even up to 400 mg/day by injection. This leads me to wonder if it is really inhibiting carbonic anhydrase at all?
I’m involved with Dr Chandler Marrs’ group on Facebook, Understanding Mitochondrial Nutrients and am very familiar with her work as well as Dr Lonsdale’s. It’s clear that thiamine has the potential to help at low risk, which is reason enough to try it in my opinion.
@remy — it is possible that carbonic anhydrase is not being inhibited in vivo — it should certainly be studied empirically. However, there is evidence that it is being inhibited in vitro. See Özdemir, Z.O., Şentürk, M. & Ekinci, D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules, Journal of Enzyme Inhibition and Medicinal Chemistry (2013), 28:2, 316–319.
hi please could the author send me a private message i really need to speak with you i have M.E fibro hEDS i would really like to talk to you i am really struggling i am on facebook or gmail
It didn’t seem to have made a difference either way to my breathing. But my PEM and fatigue totally disappeared in about 2 days. There is another key factor for me though at that is B12. I take 200mg B1 & B2 daily and if I take more that 33mcg B12 the fatigue will come back. I do not have full MTHFR but do have a few notable genetic issues along that line.
Good to know you’ve had some benefit!
If you feel comfortable completing the survey on high-dose thiamine, it would be helpful to be able to include your experience. Thanks for considering. https://forms.gle/y2ZV8yqk3wUw9rph6
Take non synthetic b vitamins only. I tolerate adenosylcobalamin the best.
I’ve tried this approach several times over the years. It’s always made me feel worse.
B-12 and particular forms of B12, on the other hand, have been helpful.
Thanks for the report. I am interested in learning more about why high-dose thiamine appears to help many people with ME/CFS, Fibromyalgia, and EDS, but makes a small share feel worse. So far, it appears that it may negatively affect people with CSF leaks or other forms of intracranial HYPOtension, as well as people with severe MCAS or active MCAS flares. Also, some people felt worse on some forms of thimaine but better on another.
How long did you take the high dose thiamine, and did you titrate up slowly? I am trying it for severe long haul Covid which is manifesting for me much like ME/CFS (severe fatigue and PEM that have left me bedbound for two years) but also with many neurological symptoms like all over tingling and numbness, twitching and jerking of muscles, tinnitus, gastoparesis, and POTS, as well as MCAS. I decided to try thiamine based on reports that in many people, long haul Covid resembles beri beri, a disease of thiamine deficiency. I prepared by reading extensively about dosing strategies and pitfalls, especially on the website hormonesmatter.com. There is much information on that site from high dose thiamine pioneer Dr. Lonsdale about the phenomenon of paradox, basically that some people feel a lot worse when starting thiamine before they start feeling better. Controlling paradox symptoms involves backing off to lower doses, titrating up VERY slowly, and making sure to include relevant cofactors. It has been a lot of trial and error and has taken me over two months to build slowly to only 400mg of thiamine HCl. I still battle mild paradox symptoms every time I increase dosage but it is manageable and subsides within a few days. I wonder how many people who have said they felt worse on thiamine may have been experiencing paradox that could be managed by the techniques I mentioned above. It does mot sound like the participants in the study were titrated up or given cofactors. Dr. Lonsdale’s belief is that the presence of paradox is actually the best predictor of successful treatment.
Oh wow! I decided to start low, but I just started with 500mg! Maybe I should lower it more.
The second, but highly technical possible link I see is:
Thiamine seems to modify an important enzyme called p53 and that has research linked to ME, Inflammatory Bowel Disease (IBD), Multiple Sclerosis, Parkinson’s Disease (all diseasses reported above in this blog to be possibly improved by high dose B1). Research papers on the link between all those and p53 can be found by Googling for ” P53″. It’s a bit too cumbersome to link all the finds.
As to thiamine and p53, two important finds are:
“Thiamine antagonists trigger p53-dependent apoptosis in differentiated SH-SY5Y cells” from “Sergiy Chornyy, Yulia Parkhomenko & Nataliya Chorna” published in Nature (one of the very best science magazines on the planet).
Apoptosis is a rather strong form of cell death so it’s rather strongly related to inflammation and anything affecting apoptosis might be important in highly inflammatory disease like ME/FM/IBD/MS/PD.
Another paper linking thiamine and P53 is:
“Chapter 11 – Analysis of the Protein Binding Sites for Thiamin and Its Derivatives to Elucidate the Molecular Mechanisms of the Noncoenzyme Action of Thiamin (Vitamin B1)” From “V.I.Bunik, V.A.Aleshin†”
Saying “Noncanonical thiamin-binding proteins of systemic significance include p53, PARP1, signal-related phosphatases, and kinases. Coupled to coenzyme actions of thiamin in central metabolism, the thiamin binding to noncanonical proteins may well provide for systemic regulation by thiamin-dependent signaling cascades, similar to those involving essential metabolites, such as ATP and NAD+.”
P53 is involved in regulating apoptosis, gene expression and protection DNA from mutations and PARP1 is involved in inflammation pathways and involved in DNA repair.
Very interesting. I’ll take a look! One thing I would note in general is that thiamine is an extremely important nutrient that plays multiple essential roles in cellular energy production. I personally think that this fact has served as an obstacle to researchers considering that high-dose thiamine may be operating as a drug through a mechanism that is entirely different from its essential role as a nutritional supplement. As research moves forward, I think it will be important to distinguish between effects seen in cases where thiamine is remedying a nutritional deficiency and effects from high doses of thiamine where there is no baseline deficiency.
As thiamine hydrochloride contains aluminum which may build up in the brain and kidneys, I choose to take probiotics and natural forms of thiamine B1, as i am chemical sensitive. When I first started on thiamine I wasn’t aware that the hydrochloride version was more like a pharmaceutical drug. I took 300mg of the hydrochloride thiamine for just 2 days and both days I suffered which extreme headaches, I have been diagnosed with fibromyalgia over 20 years ago when very little was known about it and due to my chemical sensitivity I am unable to take pharmaceutical drugs. Although I have many symptoms of fibromyalgia, headaches have never been a thing for me. This is what lead me to think something wasn’t suiting me with thiamine hydrochloride as I had changed nothing else in my diet, and when taking this version of thiamine I stopped all other supplements in order to see if thiamine helped. On the 3rd day I didn’t take the hydrochloride thiamine and i had no headache. I then decoded to start on thiamine monophosphate, which is reported to be less absorbed, but as I was aware of the need for healthy gut bacteria in order to absorbe vitamins in the intestines I just increased probiotics.
I’m only 3 days in using this form of thiamine and yesterday I had more energy than I have in the past 2 years, as my fatigue as become excessive in that time.
Not sure if this is a Placebo effect as yet and will continue with the supplementing.
As you are pondering on why thiamine helps some feel better but some feel worse I questioned whether this may be due to aluminum build up. Small amounts of aluminum are found in food, water, toiletries, cosmetics, pharmaceutical drugs etc. Therefore, each individual will have different levels in their system, with differing tolerance levels and symptoms. Therefore adding to this in mega dosing may be a possible explanation.
I love the potential oxygen connection…and the blood flow connection…and the intracranial hypertension connection come to think of it. Thanks to Jeff for doing all this work and coming up with something interesting to chew on.
Speaking of B vitamins, the one B that I’ve always noticed helped was B-3 (niacin). A good flush is temporarily calming, mildly energizing, cognitively enhancing and helps with chemical sensitivities.
Interesting. What dose of niacin seems to help?
There’s a very interesting Facebook b12 deficiency group.
The protocol means you have to use oils instead of oral b bits if possible.
But there is a dosing protocol.
First you must be replete in molybdenum, potassium iodide and selenium.
You must be replete in these before you start dosing B2. B2 is the start of the cascade that allows you to process the other vitamins and get b12 up and running
I can’t afford to apply the protocol. But there are bed bound people who have fully recovered with the protocol.
There is a big problem with adrenaline reactions which must be treated with potassium..it is quite amazing how potassium does that, when I’ve messed around with b bits.
I don’t understand the ins and outs of the protocol fully. I know the scientist behind it has presented to the omf.
I know you’re looking at b1. Just thought I’d mention this
Is taking thiamin nitrate the same as thiamine HCL ?
@nerida, Thiamine mononitrate is not the same thing as thiamine hydrochloride. However, it is easier to find, less expensive, and could perhaps be as effective. The problem is that we just don’t have as much data on thiamine mononitrate and don’t know how well it is absorbed into the body compared with thiamine hydrochloride. Before we started thiamine hydrochloride, my daughter was using thiamine mononitrate, and it seemed to have a large positive effect. We switched to thiamine hydrochloride because there were studies on its absorption and on its effectiveness, so we thought that would be a more reliable base on which to build. But future studies should certainly look at the pharmacokinetics of thiamine mononitrate and on its effectiveness compared with thiamine hydrochloride.
I recently (and accidentally) found that I get a huge benefit from 1750mg thiamine nitrate. Before trying long term supplementation, I’m first learning about the various cofactors and required dosing of those.
When on thiamine nitrate, it seemed as though every cognitive process was improved, as well as emotional regulation. Paradoxically, there was also some occasional underlying aggression/anger, but this may have been due to concurrent folinic acid mega dosing.
I’m not sure why I haven’t just ordered the necessary cofactors, I think maybe just being too cautious and also, it seems too good to be true. Motivation was increased, PEM almost non-existent, my drive to socialise was much higher, executive function greatly improved…..
Alright, time to get on iHerb and make a new order 🙂
Good luck! 🙂 Please keep us informed!
Will do, and thanks! Is there any longer term data available either from this study or from individual cases? It’s another aspect contributing to my hesitancy, that we don’t seem to hear from people having success for say 2-3 years or more.
Did you have a trial of this, Cort? I think I remember reading a comment of yours about this my not sure my recall is accurate….
I don’t remember one.
There was a subsequent study that followed up on these individuals through one year and tested impact of a lower maintenance dose of thiamine. It found no statistically significant impact of the lower maintenance dose but did find that individuals who took some thiamine between weeks 24 and 52 had lower fatigue scores than other patients, suggesting to the authors that patient self-regulation / self-medication was effective. Bager, P., Hvas, C. L., Rud, C. L., & Dahlerup, J. F. (2021). Long-term maintenance treatment with 300 mg thiamine for fatigue in patients with inflammatory bowel disease: results from an open-label extension of the TARIF study. Scandinavian Journal of Gastroenterology, 57(1), 37–43. https://doi.org/10.1080/00365521.2021.1983640.
There was another trial recently examining high-dose thiamine in patients with primary biliary cholangitis that found no impact. But it was extremely underpowered (36 patients total). As supportive as I am of randomized controlled trials, I think it is problematic to do these very small trials where you don’t really know how to interpret the findings. Did it fail to show a significant impact because it didn’t work or because the sample was too small?
While not a trial, this article summarizes the initial results of a survey I did on use of high-dose thiamine by patients with ME/CFS, EDS and fibromyalgia through Health Rising. https://www.healthrising.org/blog/2021/06/02/fibromyalgia-chronic-fatigue-syndrome-benefit-high-dose-thiamine/
I kept the survey open and then updated my analysis of the the survey results after getting 108 responses, The responses are summarized here. https://twitter.com/JeffLubell_C19/status/1638097484047163393 . And here: http://www.high-dose-thiamine.org/results-of-high-dose-thiamine-survey/
cort, i’ve taken high doses of B-complex for many yrs. is 100mg. high for B-1? i don’t know.
I really appreciated this blog, article, and information!
I have fibromyalgia and RA. In addition to significant injuries from an auto accident. The Insurance on the individual causing this “head on crash”, wasn’t sufficient to provide the necessary treatment for relief.
I will increase B-1 immediately and if you’d like will report my findings. Feel free to contact me.
Thank you again!
Yes I also would be interested in your dose please!
The Wahls protocol promotes eating organ meat as a potent source of B vitamins. You can even buy grass fed organ meat, dehydrated and in pill form.
Great article, thank you Jeffrey!
@Jeff Lubell Thank you for this informational post on high dose thiamine.
It is very wise to gather information, including doing testing to figure out if one has the problems you are pointing out, and one is suffering from thiamin deficiency. Too much thiamine can have some very negative consequences, so it’s good to know if one is solving the right problem. You do make some good points.
I’ve done very well on 750 mg of Benfotiamine daily. Now I’ve heard the folks who say that it doesn’t get into the brain, but I don’t have any mood or brain problems currently, and I think it’s worked really well and is somehow getting into my brain.
But, swayed by followers of Derek Lonsdale who banged the drum of taking specific forms of thiamine, namely TTFD or allithiamine, I decided to try their method. None of them could tell me any kind of conversion between the different forms of thiamine, so I decided to be conservative and tried 300 mg of Thiamax, one of the forms that was so highly touted.
Much to my surprise, I develop neuropathy in my hands and feet within 4 days of taking the stuff. It took me a couple of weeks to figure that out, and I dropped them and all together, and gradually added back in my benfotismin over a month, and it took the full month to reverse the thiamin caused neuropathy.
Neuropathy can become irreversible, and I was lucky to have nipped it in the bud. I’ve had a clear brain, and seem to be getting benefits from the benfotiamine I’m taking, I’d have never tried the version you discuss from the studies.
A friend suggested someone had said that people who tend to be low in glutathione, which is typical of many ME/CFS patients, who are known to have excessive oxidative and nitrosative stress, can develop this sort of neuropathy from B1.
Seems like there’s a lot to be learned about thiamine, but I do know that it has been shown to be effective in resolving the high lactate that many and me/cfs patients experience.
Thanks for sharing your experience. I do not have any experience with the fancier forms of thiamine. The research studies that I have seen on thiamine all used thiamine HCL, which is a fairly simple low-cost version. When administered regularly, within a week, it produced blood levels of thiamine that were similar to IV administration. The main difference was a lag of several days. In the survey responses, I have seen some people prefer benfothiamine and some thiamine HCL; some people report feeling better on one than the other. I am looking forward to learning more about peoples’ experiences.
Dear Jeffrey,
Thank you for all of this information! It’s so nice to have people posting who are NOT struggling with low blood flow to the brain…:(
If you don’t mind sharing, who diagnosed your daughter’s EDS and other conditions? It would be very helpful to know where to find some of the doctors that are, well, hard to find. Especially with the hypermobility version, for which there is no definitive test.
The reason for the allithiamine, from what I have read, is that it is capable of crossing the blood/brain barrier. So while other forms of thiamin might be just as effective for physical energy and other benefits, the allithiamin is better at reaching the brain for those with significant cognitive issues.
I just ordered some, so I will let you know how it goes. I will have to tinker with the dose and based on one of these posts I may wait until my glutathione levels are confirmed before I start. The allithiamine only comes in 50 mg so, rather than taking half the bottle to get to a high dose, I will add some thiamin HCI to get the “high dose” , with the allithiamin to get into my brain. We shall see.
By the way, another published paper on NIH indicated that bentofamine DOES NOT cross blood brain barrier, but of course, studies need to be repeated to confirm.
I don’t really understand the role or process of inhibition of the carbonic anhydrase, other than the effects of reducing intracranial hypertension. I am one of the few ME patients that has crazy HIGH blood pressure instead of very low BP, but it can also get pretty low…wild swings, not well controlled. I suspect due to dysautonomia. I’m sure it can’t be due to me forgetting to take meds………… Anyway, hopefully this might help with all that, too.
Have also read that it is not unusual to herx or feel worse initially with B1 (especially starting with a high dose), just like with any other medicine we might take. But the terrible neuropathy got my attention so I will just go very slow, as usual.
I will need to re-read everyone’s posts multiple times! But I want to thank @Remy for sharing how you can tell based on fizzy drinks, what a great piece of information. Thanks to all
If you feel comfortable completing the survey on high-dose thiamine, it would be helpful to be able to include your experience. Thanks for considering. https://forms.gle/y2ZV8yqk3wUw9rph6
Wow, very interesting that the TTFD caused neuropathy! I had just ordered some in hopes that it would help with my neuropathy. I just now ordered the Benfotiamine. I think I will try it first after your experience. it is a possibility that you are missing other co-factors that might have negated this side effect. Thanks for sharing your experience.
https://www.youtube.com/watch?v=-DxvSUEVT_4
Tracy, Make sure you also supplement with a really good Glutathione supplement. We use HydraStat Molecular Encapsulated Glutathione. We are on High dose Benfotiamine and TTFD depending on what we test for on a daily basis.
TTFD takes more cofactors to cleave the thiamine from other components in TTFD and it involves the Glutathione and Methylation pathways more than other forms of Thiamine, so for some people will result in what Dr. Londsdale calls “Paradox” (things like neuropathy, etc). Elliot Overton suggests building up some of these other micronutrients and electrolytes first and then taking things low and slow. More info: https://www.youtube.com/watch?v=-DxvSUEVT_4
Everything I’ve read about supplementation when you’re obviously not well is to go low and slow because of paradox – where a person gets worse before they feel better. I develop neuropathy with ME/CFS alone (I suspect thiamine deficiency now). The idea is you start off at a tolerable low dose and don’t ramp up until those symptoms resolve.
“None of them could tell me any kind of conversion between the different forms of thiamine, so I decided to be conservative and tried 300 mg of Thiamax, one of the forms that was so highly touted.“
I’m not sure what you mean by the conversion between different forms? If you ask this question on the Facebook group, perhaps Dr Marrs can answer for you. As far as I know, they don’t interconvert and have differing structures.
300 mg of allithiamine (Thiamax) is a whopping big dose. Most people start with 50 mg (or even less). Thiamine refeeding syndrome is common when starting and leads to an exacerbation of thiamine deficiency symptoms.
Symptoms from high dose TTFD can be a bit scary; it’s suggested that much of what people experience is re-feeding or paradox. Here’s a weird paradoxical reaction I’ve had since beginning TTFD. Background: in 2000 I stopped having an allergic reaction to airborne pollens (had had them since I was a kid and I was then 45). I thought it was perhaps a sign I was getting better, but two years later ME/CFS disabled (I’d struggled much of my life). This means except for one virus I’ve literally not sneezed and I used to have at least one sneezing fit every day of my life. Thiamine deficiency increases histamine. Three weeks after starting TTFD I’ve begun to sneeze again. It’s almost as if things had shut down so much that the only time I had a histamine reaction was the result of being bit by an insect or from ingesting food I’m allergic to. Now, it’s almost as if my body has enough thiamine to register a deficit. I’m hoping I’ll move through this. Neuropathy is a sign of a thiamine deficiency. Don’t know the answer to what you’re experiencing, but my own experience makes me wonder.
Also, I have glutamate/gaba issues as many of us with ME/CFS do. Thiamine will increase both glutamate and gaba and so I found myself with too much glutamate (given that I have trouble with that). Not only was I experiencing anxiety but tetany, which I’d gotten rid of the year before with magnesium. I suspected that I’d already added enough magnesium to support the TTFD and went to look for another answer. Vitamin B6 – I have mutations – I added the extra B6 (in addition to what’s in the B complex and my multi) and that’s been brought under control. I also found out in my stumbling around that B2 (yep, mutations might be playing a role) is important with both MTHFR and glutathione recycling (or whatever that’s called). Having MTHFR and MCS, I realised I needed to take extra B2. When taking TTFD, it’s important to take a B-complex, a multi, have adequate extra magnesium, support glutathione, and ensure you watch your electrolytes in general (as one or more can become depleted but particularly potassium). Lowered potassium can result in tingling and numbness. Many find they have to supplement potassium or eat some foods high in potassium daily.
I’m only about 6 weeks in on this journey and so far am managing to figure out what I need based on my symptoms.
I will comment on this as I have EDS, ME/CFS, MCAS, POTS and the list continues.
I tried the Diamox when it was first suggested as a help for both EDS and POTS. In earlier days of discovery some of us would try anything and everything to feel better. It was also said that we were to take baking soda with it as it could imbalance alkaline levels and add more dysfunction in other ways. And it tends to somewhat dehydrate you and it is a type sulfur (with methylation issues can be a potential problem). One other reason to try was, I also had the feelings of my brain being swollen and like if I could take my hands and squeeze my head and get fluid out, it would feel better. Also had a sense of pressure behind my eyes. At first it felt helpful. But then it started going wrong. I have low fluid volume due to POTS and this was a type of thing to pull off fluids. I also have low renin and aldosterone and less fluids with that problem and this being low affects the kidneys. My kidney function went into Stage 3 Chronic Kidney Disease when I did Diamox. Whether it is what contributed to it or it was just going to go that way……I can’t say. But long run, Diamox made me much sicker and my POTS did not get better, and neither did my EDS. (To fix this Stage 3 CKD, I became a strict vegan for 3 1/2 years and reversed it to stage 1 now. I’m no longer vegan, but my kidney function is holding level.) So personally, this was a failed experiment.
Now to using B1. I had tried the more synthetic version of it and find the traditional B1 is the one that helps me the most. In fact I feel it helps so much, I have told Dejurgen its one of my “must haves” of the things that are on that list of keepers. I will tell how I got to this. I tried it back in the early days of my DX with POTS as some were finding it helped. But I used the more synthetic version and didn’t find that much benefit, evidently, as I didn’t stick with it. But in the last few years I started having severe muscle weakness. I mean where I could not get up from a chair and was having to use a cane as I was so unsteady. And then I had one eye to start crossing. Just sort of go off to the side when looking straight ahead. I caught this with a selfie and showed my doctor. There are 3 things that I knew of that could cause this. One was mental illness, which I don’t have. The other was a brain tumor, which I DO have. I have a progressive menigioma that we keep a watch on. And it can cause neurological issues and personality changes…..which could had been a possible cause because of the progressive leg weakness. But I didn’t have headaches or personality changes. And the other possible thing was a lack of B1. So my Functional doc and I discussed and we both decided to address the easiest first and try B1. This has all been since COVID you guys, so I wasn’t eager to have a 45 minute lay in an MRI for a brain scan, with me having Hypogammaglobulinemia and not being able to fight off things. So I started B1, the normal type. Long story short. My leg weakness has improved to the point of my no longer needing a cane to walk. My eye doesn’t cross. I have more energy and I am better with all things. I am not well however. But B1 along with other things I’m doing is improving me. (I still have to get that MRI, as we have to keep a watch on that brain tumor. But so far, so good.)
Now as for increasing CO2. I’m doing that too but in a different way. I don’t think my taking 100 mg of B1 a day is what is doing that. (There are other supplements I credit for my improvements over all too.) But I also had severe apena. Not just obstructive but central. That is where my brain doesn’t tell me to breathe. I have been retraining myself to breathe differently. How? I use a criss cross tape that you put on top of your lips to sleep at night. When you put the tape on, you sort of poke your lips out and make sure your tongue is to roof of your mouth. Apply tape in center. (Make sure and use lip balm first.) What that does is it juts your lower jaw slightly forward. And it forces you to breathe through your nose. It also slightly increases your CO2 when you do this and it slow your pace of breathing down. That helps the CO2 to carry more oxygen into the cells. And they all work better. It helps you not have as much hypoxia. If you are a mouth breather or you snore. This will help that. I no longer have to wear a CPAP. I have a new Fitbit watch and it measures my oxygen that is on average 94% but ranges between 92% and 98%. I have good range of sleep patterns between REM, LIGHT and DEEP sleep. And it shows my breathing patterns well within normal. I have few wakes in my sleep not indicating any apena sessions. I don’t wake with headaches or dry mouth.
Now as for sleep, and I talked of this before. I MUST, MUST raise my head to sleep. It is important for the drainage of the brain to have that gravity to help that ….for me. Otherwise I have that head pressure and poor sleep. I raise the head of the bed on blocks to have the whole bed slope. And then I put another larger pillow under me to raise my head slightly more. For me with EDS the kind if pillow that gives me the most support and comfort and I can squish down into it and be fully supported is a Microbead pillow. (I like Tony Littles the best. But not cheap and there are good cheaper ones on Amazon.)
Sooooooo, long story to get to this conclusion…….B1 is one of my “Must haves”!
Thanks for sharing your experience. Very helpful! If my hypothesis about thiamine being a carbonic anhydrase inhibitor is correct, I do think that kidney function is something to monitor regularly on high-dose thiamine, just as with Diamox. I would very much like to find a nephrologist willing to put out some common sense guidance on how to minimize the risk of kidney stones from high-dose thiamine, based on their experience with Diamox. One place to start might be simply with drinking more water.
Since I take only 100mg. I don’t guess I qualify to participate in the survey. But I would say its helpful.
how much b-1 in mg. do u take a day?
My daughter currently takes 1,125 mg daily. Several of the research studies on high-dose thiamine varied dosage by weight and gender.
Have you ever gotten tested for obstructive sleep apnea and Upper Airway Resistance Syndrome?
Both lead to intracranial pressure and other neurological complications, particularly as it relates to dysautonomia, and sleeping with elevation is the 101 of managing these sleep breathing disorders.
80-90% of people with OSA are also undiagnosed. Many get misdiagnosed due to sleep studies being scored with insensitive scoring criteria too.
Hey Issie, I’m new to this thiamine thing and just did a bunch of research in the last several days. I have chronic ebv with liver and spleen pain. I have also, recently, had an intense midline pressure upon laying down (abdomen up through chest) as well as heart pain radiating into left shoulder and left jaw. The pressure got so intense 2 days ago I almost went to the ER fearing I was having a heart attack. I started thiamine 2 days ago for apnea and glucose issues and lo and behold…I am a different person today. I feel incredible! Last night…no chest pressure, my breathing changed..I could freely breathe by laying down. I am shocked and excited. The sense of doom that I’ve had for months has just vanished, literally overnight. I’m taking Benfotadine 50 mg thiamine and 150mg Benfotidine…twice daily.
So heres the part I thought important to share with you. It appears, specifically in regards to cancer, that thiamine supports health at higher doses and supports cancer cell proliferation at lower doses. So far it appears that over 200mg a day may be considered high dose but it may, in fact, be different for your type of cancer.
I currently have concerns that i have lymphoma (from ebv) . I’m currently getting labs. So I am doing some detailed reading about the use of thiamine in conjunction with cancer.
Honestly I feel I just subverted a heart attack with the thiamine so I am going to try to find the balance between supporting my heart but not agitating any possible cancer stuff. I will include the article I read about the importance of finding the right high dose of thiamine for the type of cancer you have. I wish you the best. Thank you for sharing your story.
Here is the segment from the document..its a beefy document. I’ll include the link below too so you can scan through the whole thing, if you like, for pertinent info.
“In 2001, Comin-Anduix et al. evaluated the effect of increasing thiamine supplementation in multiples of the RDI on an Ehrlich ascites tumor-mouse model [58]. Their findings indicated a statistically significant stimulatory effect of thiamine supplementation on tumor growth compared to non-supplemented controls. Moderate doses of 12.5 to 37.5 times the RDI had the greatest stimulatory effect, peaking at approximately 250% greater tumor cell proliferation with 25 times the RDI. Interestingly, at values above 75 times the RDI, no change was found in tumor cell proliferation, and a slight decrease was found at 2,500 times the RDI. This observation suggests that there is a specific range in which thiamine supports proliferation. A recent study explored the relationship between a high-fat diet and thiamine levels on the tumor latency in the Tg(MMTVneu) spontaneous breast cancer-tumor mouse model [59]. In this study a normal-fat (NF) diet contained 10% of the calories from fat while the high-fat diet contained 60%. Low thiamine (LT) levels were defined as 2 mg of thiamine per 4,057 kcal and normal thiamine (NT) levels as 6 mg per 4,057 kcal. Tumor latency was significantly longer (295 days) in animals given a NF/LT diet compared with animals on NF/NT (225 days). Interestingly,the delay in tumor latency from LT was abolished when given a high-fat diet. This demonstrates an important interplay of dietary constituents on tumor progression that needs further characterization. Although more research is needed to confirm and evaluate the role of thiamine on disease progression, these studies have significant clinical implications. First, patients requiring thiamine to treat either chemotherapy or disease-associated deficiency should receive high-dose thiamine to avoid enhancing tumor growth. Second, self-supplementation of thiamine by cancer patients should be avoided as the low-to-moderate levels of thiamine may contribute to disease exacerbation.”
Issie, This is all super helpful info. Could you tell me where you got the instructions for the mouth taping for the apnea? I also have apnea -moderate central & some obstructive. If you could share a link to the mouth taping info, I’d appreciate it. And what mouth tape you use. I use Somnifix tape with ASV CPAP machine and low flow O2.
I’ve been taking B1& B2 200mg/day for about 18 mths since I read Cort’s 2013 article and decided it was worth a try. I’d been taking Benfotamine and then found out it doesn’t ross the blood/brain barrier. Within 2 days my CFS fatigue and PEM disappeared. In the last 6 months my food intolerances have progressed to borderline MCAS. I found out that NAC can decrease DAO so I stopped that and am taking lipo-glutathione instead. If B1 decreases DAO it looks like I’ll have to experiment decreasing the B1/B2 to 100mg/day and see what happens. That’s after I get my thyroid straightened out after a recall Walmart didn’t tell me about last summer. if it’s not one thing it’s something else, isn’t it? But we are making progress thanks to Cort and so many others research now. Hang in there everyone!
Genetically, I have genes that would decrease DAO. I also have severe MCAS, in the past. I have been on the protocol to reset/rebalance my histamine receptors. I no longer use antihistamines. Occasionally use mast cell stabilizers. But use a supplement to help balance my MCAS and watch my histamine causing foods. I’m better with that than I have been in years. Not over it, but better than I was on antihistamines, they really caused me issues with my brain. My brain is back and I can research again. Yayyyyyy! And I feel better! No more swings like when antihistamines wore off.
I don’t find that 100 mg B1 makes my MCAS worse nor do I find it causing my neuropathy to be worse. But I do have more muscle strength with it and it seems more ups than downs. I do take it in mornings as it does seem to increase energy.
Also having tried a real good trial of Diamox and knowing how that made me feel in my head and my body……I can say that B1 does NOT give me the same feeling that the drug did. I don’t feel that same effect. Soooooo, ??????? Not sure if thats the mechanism of the benefit.
Can you share more about the “protocol to reset/rebalance my histamine receptors” or a link to more info? Thanks!
We have some threads on Healthrising Forum that links a book i read that is very technical. (One of the hardest I’ve read as it explains deep science of the histamine receptors and how they work.) It made sense to me. And I started trying things. I will say, as I don’t think I’ve updated what I’m using there, that I find Nettle tea and a Bee Propolis/pollen/ royal jelly to be my best helps for MCAS. Along with watching histamine causing foods or foods that I’m sensitive to which include high oxylate, lectin and nightshade foods. Dejurgen and I have written many times on histamine and its benefits. And why it is needed and not to be completely suppressed. You can search his name or my name and probably pull up some post under histamine. And then I know there is the one by Bayard on the Forum. (And where the book link is.) But Dejurgen and I have one too, but we haven’t updated it.
Thanks, Issie!
Thanks Issie! Could you share what the supplement is that you are using for mcas? Is it the bee propolis, etc stuff you mentioned below, or something else? Thank you 🙂
@mj, yes the bee stuff, nettle tea and being careful with diet. The one other diet thing I forgot to mention was I try to do no gluten.
Glad to hear thiamine has been helpful! The relationship of thiamine to mast cell issues, if any, is one area that is really not clear to me. My daughter’s MCAS has not gotten worse with high-dose thiamine, though her food tolerances have shifted around a bit. But the MCAS has not gone away as I had hoped it might. I would imagine there could be many different explanations for food intolerances getting better or worse. Certainly supplements / medications can play a role, but many people take multiple supplements / medications, so it can be hard to isolate which one is having an effect. Hope you figure out an approach that works!
We are so, so sensitive to supplements and medicines. We can use so much less than what may even be considered a “normal” dose. If people realized how few of something it takes to trigger a response, they would be surprised. Dejurgen always says, look how small an amount of pollen it takes to trigger us having allergies. Or how small an amount of gluten to trigger celiac, even a small dusting can trigger it. Since we are sooooooo sensitive, more is NOT better. More can imbalance us even MORE. Soooooo, lower and slower. Paying close attention to response and no fast adjustments.
He is Soooooooo Right on that one.
Low-Dose Naltrexone 4.5mg has helped my food intolerances & MCAS dramatically. As did starting (refrigerated) Creons for severe digestive/absorption issues, which was the first step. Then LDN seemed the cherry on top. If she hasn’t tried LDN already – and I mean, a good 6-12months stint as it took me that long to see improvements- I would highly recommend it.
Also, I am sorry to hear of your daughters health struggles, especially so young. My ME/CFS started at 18 and I am now 36 so my heart breaks for her. I so hope cures will be found in her lifetime!!
And commend you for fighting for her, your support will make a world of difference to her life.
Hi T Allen,
Thanks for the share. Can you please tell me what DAO is? And you have learned that NAC decreases it but not lipo-glutathione? (I currently take NAC and am about to start liposomal glutathione). Are you saying that B1 also depletes DAO? What the heck is DAO?
Thanks!
Kate
I also found this on B1:
“B1 is necessary for the production of hydrochloric acid, for proper digestion.”
Throughout my 26 years with ME/CFS
adequate digestion has been a problem, leading to malabsorption, etc. I must now take Betaine HCL to digest meals properly.
Interesting. thanks!
Hi I was taking 1000mg pd for approx 2 years after reading the small study and desperate to recover. I only stopped it recently because I feel ok with my multivits and celery juice regime. I no longer nap during the day unless bored or I relapse. I work part time and recently started doing 10 mins yoga sessions and I have an 8 year old to care for as a single mum. I recharge one day a week when he visits his dad but even when he is home I can manage. I still have me/cfs and I cannot do anything that increases my heart rate to much such as exercise or stress or the PEM comes. I will use B1 again if I notice my energy levels dipping because I believe it made all the difference to me. Off topic in case anyone is interested I had the Oxford vaccine with no side effects and I’d been so stressed over it!
Thanks for sharing your experience. If you are comfortable completing the survey, would be great to capture your experience.
Interesting timing. I’ve been taking this for the last few weeks. I didn’t think 200 mg was considered to be high dose, but since it qualifies I’ll take the survey.
I experienced some complications which probably won’t fit there (my apologies for the length!), but might be relevant for some of you. I’ll post here so hopefully you can learn from me before going through anything similar yourselves!
Oxalates, and oxalate dumping, and MCAS have all complicated the picture for me. I’ve experienced a strong paradox reaction – that Drs. Lonsdale and Marrs warn about – but which tells me something about B1 is on target. I take heart from their posts that the paradox reaction is a sign that things can get better.
Significantly – for the first time since I got my period several decades ago – I’m, ahem, … regular! That’s something, let me tell you!
A number of weeks ago I happened to come across a number of articles on B1, all in a short period (which at first I didn’t register as relevant to me), including:
Mercola: Ominous B1 Deficiency Found Throughout Food Chain: https://articles.mercola.com/sites/articles/archive/2021/02/08/deficiency-of-thiamine-vitamin-b1.aspx
The Ocean’s Mysterious Vitamin Deficiency: https://www.hakaimagazine.com/features/the-oceans-mysterious-vitamin-deficiency/
Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease: https://doi.org/10.1111/j.1742-1241.2011.02680.x
But it wasn’t until I got a notice for this article that everything started to come together:
SIBO, IBS, and Constipation: Unrecognized Thiamine Deficiency? https://www.hormonesmatter.com/sibo-ibs-constipation-thiamine-deficiency/
I was in a particularly bad crash at that point because of pretty strong oxalate dumping (having recently gone very low oxalate without understanding the risks) – which included a lot of pain, acidity, and a complete cessation of gut motility no matter what I tried. So the HormonesMatter article was a ray of hope. The Mercola and Hakai articles had registered somewhere, and once I put all of that together I sent my husband out to get thiamine. He got the advice that benfotiamine(sp?) was the best absorbed, so I started on that. Considering my history, I opened the capsule and took about 1/3rd.
The Good:
Holy Moly. INSTANT MOTILITY! And a feeling I could really breathe, for the first time in months (perhaps years). And I started feeling like one of the patients from the movie “Awakenings” after their first dopamine treatment. Energy, and a drive to want to do things. Why had no-one ever suggested this before?
The Bad:
But that was quickly followed by a whole mess of negative symptoms that grew over the three or four days I tried it – growing severe pain, extreme acidity, horrible gas, emotional lability (my poor husband 🙁 !), sympathetic system on overdrive (even more than usual), feeling buzzed and unable to sleep. And more ugly stuff I’m sure you don’t need to hear. Worst of all (I now realize, but didn’t notice how badly until yesterday) my hair started falling out. Severely. So fair warning to all. Not sure that last bit is exactly the fault of thiamine, but that’s when it started.
Eventually I found my way to Cort’s 2013 articles and survey (thank you Cort!). And read a few more articles on the HormonesMatter blog – including the concept of paradox reaction, which I clearly had. So I sent my poor hubby out again, this time for thiamine HCL. That made a huge difference – much more tolerable. I worked my way up to 200 mg, but that aggravated things after a while. So I have just this morning backed down again after reading more about the paradox reaction.
Once on the thiamineHCL the worst things died down, but haven’t gone away completely. Except – my flushing and histamine attacks got MUCH worse, whereas I hadn’t noticed that with the benfotiamine.
I can’t know for sure, but it seems the thiamine (both kinds) accelerated the oxalate dumping, which caused the worst of the symptoms – including through the roof acidity. Since the wonderfully increased motility has kept up (yah!) I assume my body has taken that as a signal to keep dumping oxalates. Aside from switching to regular B1 (HCL), I’ve found some things which seem to have helped that considerably, and also seem to have helped the histamine reaction:
1) I had a very (delicious!) high oxalate meal, which is known to break oxalate dumps for some people.
2) magnesium oxide, alongside my usual magnesium citrate, is highly alkalinizing and that helped immediately;
3) evian water is one of the lowest acidity waters (much better than our city tap water – despite the fact we filter it) and seems to be helping quite a bit as well. Hubby has just gone out to look for some alkaline water to see if that does anything.
4) taking the B1 very early in the day helped with the WIDE-AWAKE-INSOMNIA (still there, but much better), and
5) taking it together with magnesium oxide, quercitin, potassium and vitamin C seems to have also helped damp down any histamine reactions (still there, but brief and much weaker).
So, overall my motility now is a thing of magic(!), I have more energy (not huge, but there), and feel like I can breathe easier. (But not anywhere near as well as the first day).
The scary thing is the pretty extreme hair loss (in just a couple of weeks), which is probably due to oxalate dumping, but is known to be caused by low B7. So I’ve sent my husband out to get some biotin (b7) and alkaline water. Hoping to heavens that works.
My apologies for the long essay. Still trying to put all this together, and didn’t think most of this would fit on the survey (or make much sense), and wanted to give people a heads up to the possible downsides.
Sorry – brain fog strikes again. Jeffrey was commenting on the vaccine survey in the comment above – somehow I interpreted that to mean that Cort was doing another B1 survey!
You might be right on about needing the biotin. The Thiamine supps may be competing with it. Might consider taking the Biotin away from the Thiamine?
That too fast oxylate dump….ugh! I’m sooooo glad you warned of it. I knew to slow that down when I went lower oxylate. It was a BIG thing that has seemed to help with me, getting oxylate diet lower. And is NOT to be done in a quick way or there is some really nasty side effects.
As for hair loss, I have a friend in the business of hair restoration and she says that we have hair turn loose 3 weeks before it starts to visibly shed. So think back 3 weeks ago and see what changed. It could had been an emotional upset even.
I too found that the regular form of B1 was the most effective for me too. I took bottles of Benforthimine and didn’t see benefit, years ago. But B1 HCL does make a difference.
Low and slow when changing diet to low oxylate.
” And a feeling I could really breathe, for the first time in months (perhaps years). And I started feeling like one of the patients from the movie “Awakenings” after their first dopamine treatment. Energy, and a drive to want to do things. Why had no-one ever suggested this before?”
Especially with the “a feeling I could really breathe, for the first time in months (perhaps years)” I suspect a lot of the symptoms could be related to a former years to decade long situation of hypo perfusion (hypo oxygenation) in many many parts of your body being reversed far too quick.
That could IMO create something *resembling* reperfusion injury in many of those areas. The damage (and inflammation) of reperfusion injury after hypoxia / ischemia is often said by doctors to be worse then the damage (and inflammation) of hypoxia itself.
If that would be the case, you might going WAY too fast to such high doses. *IF* your body would have adapted too and found workarounds for plain surviving in very low oxygen supply situations for decades, you can’t expect it to do well when you flood it at once with oxygen again.
Compare it to having a house with all doors and windows closed and with a smoldering fire and plenty dark smoke in it. Open all doors at once and many chances you have either a ball of fire hurled your way or at least flames flaring up massively.
Just like that flaring flame isn’t a sign everything will be better soon, the same thing could be said for that (if present) reperfusion injury inflammation.
I use another trick that improves my breathing a lot. It took quite a bit of puzzling to get it start working without backfiring and the patience to grow from ridiculous small amounts of supplement to still far smaller then what the label says a year later.
Issie, who has more genetics issues then I do, isn’t able to get it working even after two years and her being utmost sensitive and able to get unlikely things working. The trickyness of this one with the big potential to backfire is why we don’t share this one. But for me: going loooow and sloooow helped me so much.
With 500x daily recommended dose this strategy might seem impossible, but for example starting 1x, 5x, 10x, 20x,… at 1 step per month will get you there in less then a year too with the potential for underlying inflammation being cleaned up by given the right doses of extra oxygen needed for optimal repair rather then being flooded by it at once.
Thanks for sharing both your positive and negative experiences. If you feel comfortable completing the survey on high-dose thiamine, it would be helpful to be able to include your experience. Your remarks here are also helpful for expanding on your experience. Thanks for considering. https://forms.gle/y2ZV8yqk3wUw9rph6
Jeffrey:
Thank you for your article, and for doing this. I hope all of this will prove helpful for your daughter – and the many people who share her conditions. Thank you from all of us!
So there is a survey! My brain has been especially addled lately, so thanks for clearing that up. It may take me a day or so to respond; I’d prefer to wait for a time my brain is clearer so I don’t ramble as I did yesterday.
Issie:
Thank you for the comments re: reducing oxalate slowly, and the hair information! Three weeks prior would have been exactly when I started the benfotiamine; I can date it exactly because of the HormonesMatter article. I started it on March 17th. I attended a birthday party on April 2nd (family) and in those photos there is no hair loss yet (at least not visible, and I hadn’t noticed hair falling out at that point). Does your friend hold out any hope for whether or not it will regrow once the ‘shock condition’ is removed? (I may not want to know the answer if it’s bad.) What’s become obvious now that I can clearly see my scalp is how consistently crimson my scalp is. Perhaps that inflammation dejurgen was mentioning? Oddly, my face only periodically flushes, but it seems my scalp is on permanent flush?
It seems you and I may have very similar issues going on! I know that I need to take much less than the standard dose of anything (or much much more sometimes – hard to know beforehand). I’m a bit shocked that 1/3 of the pill over three or four days would do that much (but shouldn’t have been, in retrospect).
Would you happen to know of any good articles on dumping – specifically what the harms/risks are? I see lots of warnings, but none with any specifics, especially no details of what harms are likely/possible, and aside from the extreme discomfort what actual physical harms are occurring, nor what the actual danger might be.
Because of so much brain fog I find all of this both confusing and exhausting. There seem to be quite a few people writing about oxalates, and thiamine, etc… but – from my perspective – it seems they’ve all forgotten how their brains worked in the midst of their disease states. Or perhaps they’re all still in that state a bit? So I’m finding it’s too easy to miss important details and considerations, and even sometimes the overall picture. I wish someone would spell out the overall picture and the most important things in point form so I know what’s important to attend to.
Cort:
Which reminds me – thank you Cort for including ‘The Gist’ in your articles. That’s SO helpful when my brain isn’t working. I wish more people would do that when they’re writing for people with cognition issues.
dejurgen:
Thank you for your detailed explanation, and especially the very clear cautions about reperfusion injury! I’ll certainly take that on board. So the movie I should have been referencing was ‘Backdraft’? 🙂
I wasn’t clear on exactly what it was that worked for you but not Issie re: improving your breathing. Was it that you were able to start out at a very low dose of B1 and slowly increase to a high dose, but that Issie needed to remain at a low dose? It sounds like Issie and I have many of the same challenges, so if so that is good information. If I’ve misunderstood, would you mind explaining a bit more?
Thank you all!
@Anne:
“I can clearly see my scalp is how consistently crimson my scalp is.”
I’m not a native English speaker, so I miss the subtle definition of what color is exactly described by crimson.
If it’s red without any blue, black or brown-ish hue, then there is a good chance it points to rather good blood flow with a possible touch of inflammation. If your hair falls out, their might be more inflammation going on then you desire. With good blood flow, lack of blood flow won’t be the likely cause of hair loss.
As the scalp is one of the places where blood reaches slower to, it sort of would suggest that much of your skin everywhere should be well fed with blood and full of color if it where just that. As you say your face only flushes periodically, it could well be that you have an MCAS / allergic reaction going on on your scalp. Is it rather itchy?
The thing that helps me with better breathing isn’t B1. It’s an OTC thing that IMO has quite a bit more punch then B1 in select cases and that includes quite a bit more potential to fire back dangerously strong. We both experienced it. We will disclose that one later but only with massive warnings, more to show what mechanisms it seems to modify with us.
@Anne, do you have alopecia and hair coming out in clumps or is it more of a shedding? Alopecia is considered somewhat autoimmune and that is unpredictable as to whether it comes back or not. I have it occasionally and when I lost one large clump, Mayo doc gave steroid injections into the spot to stop the autoimmune attack. It did come back, but I also have vitiligo (lack of melanin/color pigment) and it came back in a most beautiful silver. And now with some additional silvers coming in, my hair looks beautiful streaked. I wanted more of them put in, but the beautician didn’t think she could match the color well enough. Told me to give it time and it would happen on its own. LOL. I embrace it. I like it. I’m mostly an ash dark blonde with these beautiful silver streaks. But I also have had excessive shedding of my hair too. I think that is stress related and just generally being in a Chronic health state. That is unpredictable as to whether it comes back in too.
The low oxylate diet and being slow to detox to not make yourself have to bad of a dump…….maybe Dejurgen will chime in on this one. Me warning him to not stop all oxylates immediately as this could happen. He didn’t feel none too good…..as he went to fast. We both still do occasionally eat oxylates. But we know we feel better when we don’t and limit them. Its hard to avoid completely. But there is an app that you can look up oxylate levels of foods and make better choices. It has helped my pain and taking down inflammation and my gut is better. It can also help liver function for better function. Many nightshade are high oxylates too, and those really make me hurt. Eliminating gluten has helped and for sure lower lectins. Beans especially are a problem for me. I also have problems digesting red meats.
Hope this helps answer some questions.
And as Dejurgen said, face flush…..look at MCAS.
“Would you happen to know of any good articles on dumping – specifically what the harms/risks are”?
https://www.youtube.com/watch?v=RdFOSBwFTaM
Thank you Ann for all this information. I am actually going to come back to this page in the morning so I can take NOTES.
I will just add that the hair falling out en masse is a very typical symptom of hypothyroidism. I’m sure it is a symptom of other things too, but I’m wondering if there might be some interplay between B1 and thyroid, or just hormones in general.
I’m going to have to go read your links on oxalate dumps. This is a new topic for me, so thank you.
This will be another rather technical plausible explanation. This new idea now makes most sense to me so far.
There is plenty of information to be found on “thiamine deficiency alcoholism”. The correlation has been found many times. The why, that is a lot harder to find. Hence the search.
There are a few papers reporting thiamine levels dropping fairly clear and fast after alcohol ingestion. That indicates it’s not a longterm effect after months of alcohol abuse, but thiamine seems to get consumed at high rates after each alcohol overconsumtion.
See for example the paper with title “Thiamin depletion after ethanol and acetaldehyde administration to rabbits” by M Takabe, Y Itokawa
Some researchers pointed to acetaldehyde, a waste product of alcohol consumption, being more important then alcohol itself in the loss of thiamine.
Then, I did found that pyruvate can be converted into acetaldehyde by a substance called hydroxyethyl-TPP and TPP is the abbreviation for thiamine pyrophosphate. Thiamine pyrophosphate is a phosphorylated form of thiamine. In biology, many things are phosphorilated to become active.
So a chemical called hydroxyethyl-TPP that can convert pyruvate to acetaldehyde is only a single modification away from the common active form of thiamine, thiamine pyrophosphate.
When adding B1 or thiamine, one doesn’t want to produce more acetaldehyde as it is a rather toxic chemical in too high quantities. So the two main questions are:
A) Would the reaction be able to go both ways, from left to right and from right to left?
B) Would that reaction go from more acetaldehyde to less acetaldehyde in humans? In other words: would enzymes that can do such also be present in humans rather then only in specialised bacteria or such?
The answer was (after a difficult search) hidding in plain sight:
Wikipedia(Pyruvate_decarboxylase), first picture on the right hand side https://en.wikipedia.org/wiki/File:Pyruvate_decarb_1.svg
=> Pyruvate decarboxylase also has a less well known reaction (aside from its involvement in/near the Citric Acid Cycle) between pyruvate and acetaldehyde and it goes in both ways.
=> It can, from the middle, convert acetaldehyde + CO2 into pyruvate.
=> It uses TPP, or thiamine pyrophosphate and Magnesium as cofactors; both are frequently said to improve ME/FM/…
=> Pyruvate decarboxylase is a profoundly human enzyme, so we are not talking about an esotoric enzyme only found in rare bugs. We have it and it is vital to our survival!
=> (high dose) Thiamine offers the potential to speed up acetaldehyde detoxification up a lot.
Acetaldehyde is a rather toxic and reactive chemical that is known to increase RBC stiffness. So reducing excessive acetaldehyde levels can reduce RBC stiffness, a commonly reported research outcome in ME patients. Too stiff RBC impair blood flow and oxygenation. Jeffrey mentioned better blood flow and oxygenation as main benefits in this blog.
AcH Influenced was one of the pioneers on this topic and wrote about it in https://www.healthrising.org/forums/threads/measured-high-endogenous-acetaldehyde-blood-levels-discovered-something.6126/
Issie and I are also developping strategies to reduce excess levels of acetaldehyde. Warning: lowering acetaldehyde levels too much and or too fast can create severe drops in dopamine levels (as acetaldehyde increases dopamine levels).
When searching for the combo “thiamine dopamine” one can find several papers on their relationship. An interesting paper is titled “Vesicular dysfunction during experimental thiamine deficiency is indicated by alterations in dopamine metabolism” by “D D Mousseau 1 , V L Rao, R F Butterworth” saying:
“thiamine deficiency… …other regions also known to be involved in sensory processing and intellectual function (e.g., frontal cortex, hypothalamus, thalamus), but having a greater noradrenergic input, had increased levels of 3,4-dihydroxyphenylacetic acid (DOPAC) and decreased levels of other dopaminergic metabolites including noradrenaline.”
Have we got a winner here?
I would say……YES, WE HAVE A WINNER!
So, this is a connection to what we have been working on with acetaldehyde……
And as Dejurgen said, don’t lower acetaldehyde too much…..it was a major disaster when I did and lowered my dopamine too much. (Yes, that lab rat experience, went not so good.)
B1 is a keeper for me.
Edit: in my fatigue searching this, I confused pyruvate decarboxylase with another enzyme. But my luck, it seems to be present in humans too as there is a rare genetic disease called ‘pyruvate decarboxylase defficiency”.
See https://rarediseases.info.nih.gov/diseases/4620/disease
“Pyruvate decarboxylase deficiency”
By the name of the link I believe this is a genuine NIH site and as such should talk about humans. If humans can have a diseasse called
“Pyruvate decarboxylase deficiency” then I can only imagine that healthy humans do have the enzyme “Pyruvate decarboxylase”.
Note: acetaldehyde is a common byproduct of excessive oxidtaive stress inside cells. No need to drink alcohol in order to have too high acetaldehyde levels.
Alcohol, getting converted to acetaldehyde before it is finally detoxed is a common source of trouble for ME patients too.
And certain pathogens and yeast can also produce acetaldehyde.
As Dejurgen said, no need to drink alcohol for it to be too high. We could be our own producers of it with what is within.
I remember reading once that alcohol intolerance was seen as a significant marker of ME/CFS…
“=> Pyruvate decarboxylase also has a less well known reaction (aside from its involvement in/near the Citric Acid Cycle) between pyruvate and acetaldehyde and it goes in both ways.
=> It can, from the middle, convert acetaldehyde + CO2 into pyruvate.
=> It uses TPP, or thiamine pyrophosphate and Magnesium as cofactors; both are frequently said to improve ME/FM/…
=> Pyruvate decarboxylase is a profoundly human enzyme, so we are not talking about an esotoric enzyme only found in rare bugs. We have it and it is vital to our survival!
=> (high dose) Thiamine offers the potential to speed up acetaldehyde detoxification up a lot.”
Now you’re touching on my question regarding coenzymated B1, known as thiamine pyrophosphate (TPP) or cocarboxylase.
I’ve been taking Source Naturals Coenzymated B Complex for years. A nasty GI viral infection that triggered Guillain-Barre also wiped out many of my parietal cells, and I developed severe anemia as a result. The coenzymated B vitamins did the trick and corrected my riboflavin and B12 deficiencies, and the anemia was resolved.
I wonder if this is why we need such high doses of thiamine in ME/CFS. Perhaps we lack parietal cell density and cannot adequately convert thiamine.
So that has me wondering if anyone has tried taking TPP/cocarboxylase in higher-than-typical doses in lieu of high doses of thiamine hydrochloride.
@judi lane, were you checked for h pylori? As that can wipe out parietal cells too?
Also, my dad had Guillain-Barre and anemia too. His was also a lack of B12. (And I have a cousin with your name. I’m going to text my cousin and see if this is you.) But his Guillain-Barre was from a flu shot, they thought. But I have issues with h pylori and me and my dad are/were a lot alike. If this is another cause of your parietal cells being depleted, I’m finding Mastic Gum to be helpful. I’ll look into this type B1, I have not tried it. Thanks for your comment!
Not H Pylori. But it does make me wonder if coenzymated thiamine would work as well or better.
Just a little caution, it is tricky to add one B vitamin out of proportion to the others.
For example, B2 is often thought to help prevent migraines, esp in combination with butterbur and CBD oil. And Biotin is thought to help a variety of things. And Folic Acid, etc etc etc.
We just need to keep cognizant of balances.
While we are talking about FM and Me and pain etc, I’d like to point out how helpful the Omega 3-6-9 acids are. But again, in their proper proportions. Olive oil is heavy in omega 9. Fishes are heavy in omega 3. But we also need proper proportion of Omega 6 for control of inflammation. Primrose oil and hempseed oil are good sources.
It is tricky to start playing around with nutrients without keeping the big picture in mind. This includes the B vitamins. They have to be in balance with each other.
Wow, lots of interest in B1!
I’m in the ME/CFS and EDS camp. I suggested to one of my EDS friends with progressive small fiber neuropathy, that B1 was found to be helpful in treating that. Not sure if he took me up on my suggestion. Later I tried it myself, but cannot remember what form or what dose–but don’t think it was as high as recommended in this blog. I don’t recall any improvement.
Currently I take Pure Encapsulations B6 Complex which has 100mg B1 along with the methylated versions of other Bs–especially folate since I have MTHFR. Maybe I should try B1 again at a much higher dose.
I am currently noticing some energy improvement using higher dose N-L-Cysteine and dividing my Hashimotos meds to a different ratio of T4 to more T3 (I’m euthyroid but have hypo symptoms).
Issie, I’m blown away with your list of medical issues. You won the genetic lottery! I’ve got less but make up for it with achalasia. Glad you are finding stuff that works!
@Nancy B, that’s what Dejurgen says of my genetics……I sure wasn’t handed a very good set of cards. But we find that can be true for many, just people are not aware of that. Thats why its a very individual journey and why not everything will work for everyone. But Dejurgen and I are finding work arounds, even with my wonky genetics and I’m making forward advances. Not as fast as he is, but any forward movement is another step in the right direction. Doesn’t matter what we get handed…..we can improve.
Sorry about your swallowing issues. My hubby has that and has found it is extremely important to read labels and have absolutely NO GLUTEN. We found that was his trigger. He was DX with Barrett Esphogus, but has totally reversed any signs of it with being strict on gluten or anything that the immune system can interpret as gluten proteins. It really is a most miserable thing to have! Hoping this may help for improvement for you too!
I tried large IV daily injections & after a couple months YES I started to feel normal .. I had energy to enjoy life BUT sadly I ended up with horrible adult acne that has taken a year of treatments to fix my skin .. I was so excited to feel normal again but the ugly & sore acne on my face was too much to handle … hence I needed to stop ! Lower doses didn’t help my CFS/ME.. I am 64 female maybe it could be different for others .. as I always live in HOPE!
Thanks for sharing your experience. Sorry to hear of the side effects. If you feel comfortable completing the survey on high-dose thiamine, it would be helpful to be able to include your experience. Thanks for considering. https://forms.gle/y2ZV8yqk3wUw9rph6
Might consider Pantetheine (B5) for the acne. As we all know, when introduce one B vitamin at high doses & it can disrupt other B vitamins. It might be a possibility that you could tolerate the Thiamine, if you oppose it with some Pantetheine?
Wow what a response above. I have not read it all as much of it is out of my league.
However i would add that on online user/advocate for Thiamin is Izabella Wentz. She is in the thyroid/Hashimotos space. She swears that is shifted some of her symptoms.
Perhaps there is something in her use of the vitamin that may provide some ideas on the mechanism of how it may have effect.
Can any one supply a link to the thiamine HCL they take (not the Benfotamin) please? I’m in the UK. I’d like to thank you so for this info. I’m going to try it, albeit slowly and build up.
I’m just curious which brands people would recommend.
This is the one we have been using in the U.S. https://www.gnc.com/vitamin-b1-thiamin/259513.html
I looked at the ingredients in the brand you are using. Its very high in dosage and some of the fillers could potentially cause some people MCAS issues. Some, not all MCAS people have issues with microcrystaline. I wonder could that be contributing to your daughters MCAS? We have to be careful with our fillers. Some people react to rice too and this has rice powder as a filler. Like I said in an earlier comment, even a light dusting of something we are sensitive to, can cause a problem. And MORE is not always better. For us sensitive ones, MORE can mean more problems. Lessor is More and better many times, and usually, in my case.
G&G here in the UK do a version with no fillers and in 100mg or 500mg doses
I use 100 mg.
I have just ordered Solgar vitamins, 500mg. I am in the UK.
Thank you Lemnia for the question, and Issie for your response.
I checked, and the B1 HCL my husband bought (Natural Factors) does indeed have microcrystaline cellulose as one of the fillers. I’m kind of shocked how much it’s aggravated my MCAS! Makes me hopeful I’ll be able to find a version that’s cleaner.
Jeffrey Lubell, I read this post and your blog post on medium.com. EDS is a fascinating thing, and I believe some cases are caused or exacerbated by Bartonella infection. If you haven’t read the works of Ed Breitschwerdt, please do us all a favor and dive into it. Two links at the bottom of my post.
My second point is my own thinking – is EDS can be caused by Bartonella species infection, and it is difficult to clear this infection, as it is with Lyme, maybe somewhere in the patient’s biochemistry, the infectuous organism is playing a part in the biosynthesis of the certain vitamin and/or mineral deficiencies, or modified/modifies biochem cycles. Or maybe parasites are just stealing the stuff, and we don’t appear to have deficiencies, but we are not getting the levels of nutrients we need.
One last thought of my own: EDS is said to be genetic, but perhaps it’s Bartonella that modifies genetic expression, and/or can be passed from mother to baby?
Rheumatological presentation of Bartonella […]
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5944489/
Ed Breitschwerdt
https://cvm.ncsu.edu/directory/breitschwerdt-ed/#tabsPnl1-tab-2
Thanks for sharing the links! I’ll look forward to reviewing.
I’m not sure my experiences on thiamine will be helpful because it is a confusing story. Years ago my integrative medicine doctor recommended high dose thiamine for my ME/CFS. I think it was 400 mg/day. I don’t remember any positive effect. Later I started taking benfotiamine 250 mg twice a day – I don’t recall any positive effect. Now I’m taking 250 mg benfotiamine, not sure why.
Another possibly related issue is that for a while my glaucoma was out of control and my eye doctor prescribe Diamox to lower my eyeball pressure as a stop gap before my glaucoma surgery. I felt pretty yucky on that stuff. I wonder what that says about the possibility that high dose thiamine might help my fatigue?
Rachel, an important part of the survey is understanding for whom high-dose thiamine works or doesn’t work. If you have ME/CFS, Fibromyalgia or EDS, and feel comfortable responding to the survey, please do so. Thanks for considering.
If 83% of people with ME/CFS have intracranial hypertension, that sounds pretty bad. I looked up how to diagnose it – brains scans (MRI or CT) and a spinal tap. Spinal tap sounds invasive and not necessarily safe. Is there any point in trying to get a diagnosis? Do other people with ME/CFS try to get diagnosed and treated? Does treatment help? I’d love to find out more. Thanks!
Hi Jeffrey. This is a really thought provoking blog. Thank you.
Your blog suggests that issues transporting thiamine from the blood stream to the mitochondria could feasibly cause the PDH block theorized by Fluge and Mella, at least in some patients. A PDH block will affect the citric acid cycle and myelin and acetylcholine production. Acetylcholine certainly appears to be low in many patients and I feel it is the thread that unites most illness subtypes, including autoimmune.
It is interesting that your daughter has cervical instability, which in some seems to be the precipitating cause of the illness and a fixable and curable cause, but in others appears to result from the illness? I wonder what the biochemical or electrical link is between the cervical instability (and by extension the CNS) and thiamine and PDH? Some form of two way signaling must be disturbed that does not occur when the spinal cord is completely severed as in paralysis.
All the best to your daughter and with your research. Is an interesting topic.
Thanks for your thoughtful comments. One of the intriguing and challenging aspects to studying high-dose thiamine is that there are so many different potential mechanisms to explore. With thiamine playing a central role in aerobic respiration and proper mitochondrial function, much emphasis has understandably been placed on those pathways. The passive transport theory of thiamine seems compatible with improvement in mitochondrial function related to the hypothesized PDH block. Alternatively, the outcomes could be explained by thiamine’s role as a carbonic anhydrase inhibitor — for example, mitigating tissue-level hypoxia and clearing lactate. In this case, high-dose thiamine is compensating for, rather than correcting, an underlying problem.
On another topic you raise, Jeff Wood suggests that brainstem compression related to cervical instability may be at the core of at least some of the challenges faced by some people with ME/CFS. https://www.healthrising.org/blog/2021/02/11/could-myalgic-encephalomyelitis-be-a-chronic-ongoing-traumatic-brain-and-spinal-cord-injury-which-is-exacerbated-by-exertion/
I think the vagus nerve might be affected in cervical instability . It travels from the cranium (as the 10th cranial nerve). Its functions are mainly autonomic- check out the autonomic nervous system. You will then understand that POTS is an autonomic-dysfunction clearly affected by thiamine. Has anyone tried getting the high dose thiamine by intramuscular injection? How frequently is a 100mg injection required in a week to get the same high dose equivalent to 2g daily of the tablet or capsule?
@Enny– Dr. Costantini’s team has set up a website for the use of high-dose thiamine in patients with Parkinson’s Disease.
If you go to the website — https://highdosethiamine.org/ — and search on the page for intramuscular, you’ll get his team’s answer to your question, which is that one 100 mg IM injection per week is equivalent to 2,000 mg daily of oral thiamine HCL for a week. There are also warnings on the site of occasional allergic reactions to the shots and a warning against using the shots with people on anticoagulants. I should also note that the doses Dr. Costantini advises for people with Parkinson’s are much higher than the doses he advises to treat people with other neurological conditions.
@Judi Lane:
Thanks for replying above. Your questions got me thinking and searching.
From what I know and recall, many B vitamine have both a non-phosporilated and a phosphorilated form. If I am right, the phosphorilated form gets first converted in the gut to the non-phosphorilated form, then absorbed into the gut cells.
From “Thiamin, Martin Kohlmeier, in Nutrient Metabolism, 2003, Digestion and absorption”
“Absorption of microgram amounts from the small intestine may be nearly complete; it increases with thiamin deficiency… …and decreases with folate deficiency. Aging… …and alcohol intake… …decrease fractional absorption; concomitant alcohol ingestion may decrease absorption from a single thiamin dose by nearly 30%. At doses around 5mg/d absorption becomes very ineffective…. Only about 5% of a 50 mg dose is absorbed….
So under normal conditions, when taking mega doses of thiamine then blood thiamine levels should vary way way less then the difference in intake suggests. There are remakrs to be made:
The small bowel has way more surface area then the diameter of the tube suggests. It has plenty of “protrusions” called Wikipedia(Intestinal_villus) that increase the surface area for absorption vastly. Inflammatory (gut) diseases, not uncommon in ME/FM/… can:
“Villous atrophy
In diseases of the small intestine the villi can become flattened due to the effects of inflammation, and the villi can sometimes disappear. This deterioration is known as villous atrophy, and is often a feature of coeliac disease.”
That would decrease the ability to absorb any nutrient including B1 a lot, creating an actual deficiency. Mega dosing might overcome this problem.
Still, Jeffrey commented
“When administered regularly, within a week, it produced blood levels of thiamine that were similar to IV administration.”
So this suggest elevated blood levels of thiamine “overcomming” this poor thiamine absorption at high dose (even in healthy people). Note: would you have a reference paper to this, Jeffrey?
Sure. Smithline HA, Donnino M, Greenblatt DJ. Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects. BMC Clinical Pharm 2012;12:4 (2012) https://link.springer.com/content/pdf/10.1186/1472-6904-12-4.pdf
Jeffrey did you come across much information on oxythiamine the antivitamin? I’ve often wondered if that is the mechanism by which thiamine works.
Interesting. I’ll take a look.
I’ve just come across something else – DRP1. Prusty made mention of this protein on his twitter feed and it is also cited by Gerwin Morris. Thiamine mediates mitochondrial fission through the inhibition of DRP1 phosphorylation. I don’t understand the exact mechanism, it hurts my head…but it seems like thiamine prevents mito shrinkage.
This search lead to an interesting new plausible method of thiamine use by the body. The clue was a paper called “Thiamine diphosphate reduction strongly correlates with brain glucose hypometabolism in Alzheimer’s disease, whereas amyloid deposition does not” by “Shaoming Sang, … …, Chunjiu Zhong”
That may point to thiamine shortage playing a big role in the Citric acid cycle. I did try and read that different however. It was Thiamin *DI*phosphate levels in the blood that correlated so well with AD.
Why is that *DI* in DIphosphate so important? By what I know, that is a form that likely needs to be converted to thiamine by dephosphorilating the vitamine twice to transport it well in the cells, where it needs to be phosphorilated to TPP (thiamine pyrophosphate) to become active again. So why is DIphosphate, a substance being IMO rather hard to transport into cells without dephosphorilating, the important thing here? Maybe the better question is: why does the body produce thiamine DIphosphate from the absorbed unphosporilated thiamine at all?
This may be the answer Wikipedia(Thiamine-diphosphate_kinase):
“In enzymology, a thiamine-diphosphate kinase is an enzyme involved in thiamine metabolism. It catalyzes the chemical reaction
thiamine diphosphate + ATP ⇌ thiamine triphosphate + ADP”
This may seem nothing unusual, but it might be very much and key:
=> It is a reaction going from left to right and right to left, so both ways.
=> More important, it is a reaction *that can and will transport ATP through the blood*!!!!!!
Remember Ron Davis being puzzled that our cells throw out ATP out of themselves into the blood, even if they have a very hard time producing it themselves. He called it unseen in any other disease and highly exceptional.
Since, I’ve written that it might make sense in hypoxic situations, where the cells produce plenty and plenty of lactic acid. That creates a very harsh environment to the RBC as those can *only* produce ATP in an anaerobic way since they have no mitochondria (allowing them to be small enough to be squeezed through capillaries).
Their glycolysis (conversion of glucose to pyruvate + 2 ATP) isn’t hindered as such, but they need to convert excess pyruvate and NADH to lactate and NAD+ or they’ll lack NAD+ needed to further convert any glucose to pyruvate and ATP. It is hence the strong increase in lactate that severally limits their ability to produce ATP needed for both their functioning AND their defence against oxidative stress. In a hypoxic environment, oxidative stress is rather high.
So, my hypothesis was that cells (with mitochondria) shed some of their very hard won ATP so that RBC could survive.
ATP however might not be the most stable thing to dump in the bloodstream in order to try and save the RBC. This *might* prevent cells that are far away from the RBC in need and that are well fed with oxygen to donate some ATP, leaving that hard task of producing and donating ATP to cells near the RBC in trouble. Those cells near the RBC in trouble however are lacking oxygen too.
So how to possibly solve this problem? Well, thiamine-diphosphate kinase allows far away well fed cells to produce ATP, use it to convert thiamine diphosphate + ATP into thiamine triphosphate + ADP and let this thiamine triphosphate “float” long distances to where it is needed. Compared to RBC, thiamine triphosphate is a very small molecule easily reaching places and passing through constricted capillaries that unflexible RBC have very much trouble to move and not be stuck.
Then in those zones with very few oxygen and very high lactate, cells including RBC can pick up that thiamine triphosphate and use an (“used”) ADP of their own and convert it in thiamine diphosphate + ATP.
With an enzyme going both ways without the need of any regulation or extra energy input, this process would almost go automatically and “spontaniously” release ATP near sites who lack ATP (or better said, who have excess or “used” ADP).
Even better: this reaction back an forth does not induce a single loss in energy (no overhead) needed for the conversion, so it’s very efficient and far more efficient then for example anaerobically using glucose (which has a cost of 6 ATP to the liver for each 2 ATP being produced anaerobically at the site of hypoxia, leading to an expense or overhead of 4 ATP for every 2 ATP provided.
=> Does tripple phosphorilated thiamine equals very small energy dense ATP rescue packages???
If so, this mechanism may also increase our absorption rates of thiamine to those far above healthy people for once possibly explaining
“When administered regularly, within a week, it produced blood levels of thiamine that were similar to IV administration.””
despite gut absorption rates of thiamine falling very hard at high doses.
How? in ME/FM/… people where plausibly this mechanism is upregulated a lot, for example by upregulating this thiamine-diphosphate kinase a lot, thiamine and TPP might be converted rather quickly to thiamine-diphosphate and thiamine-triphosphate. As such, the gut cells wouldn’t see much “regular” thiamine or TPP in the blood and feel there isn’t too much of that in the blood. That *might* allow them to keep absorb thiamine whereas in healthy people it would be blunted very very much.
That also could explain why people with ME/FM/… demonstrate signs of thiamine deficiency while there is enough in their blood (if blood test also detect thiamine-diphosphate and thiamine-triphosphate as “thiamine”): the body may use the bulk of our thiamine reserves as “ATP emergency carriers” leaving very few “regular” thiamine and TPP for (other) biological process to be used hence creating an actual deficiency inside the cells.
A quick search on thiamine triphosphate didn’t gave much biological processes it was involved in directly.
Looking at Wikipedia(Thiamine_triphosphate) I got
“Thiamine triphosphate (ThTP) is a biomolecule found in most organisms including bacteria, fungi, plants and animals.”
So most organism do have it and hence produce it and hence produce the enzyme to convert thiamine diphosphate to thiamine triphosphate but it is hard to find a single biological reaction that is affected by it in research? I don’t believe all those organisms encode this enzyme in DNA and produce it actively day by day “for fun”. Unless other roles for thiamine triphosphate are to be discovered, that leaves room for it as “emergency relief ATP carrier”.
“It has been proposed that ThTP has a specific role in nerve excitability,[2] but this has never been confirmed and recent results suggest that ThTP probably plays a role in cell energy metabolism.[1][3] Low or absent levels of thiamine triphosphate have been found in Leighs disease.[4] [5]”
OK, so reasons to believe it is involved in nerve excitability and cell energy metabolism, but so far no single biological reaction “caught” doing so?
From Wikipedia(Leigh_syndrome), the list of symptoms of ThTP defficiency remakrably resemble many things we have and even more so during bad episodes. For me very bad episodes of a feeling of severe lack of oxygen in the air no matter how much I breathe goes hand in hand with
“As the disease progresses, the muscular system is debilitated throughout the body, as the brain cannot control the contraction of muscles. Hypotonia (low muscle tone and strength), dystonia (involuntary, sustained muscle contraction), and ataxia (lack of control over movement) are often seen in people with Leigh disease. The eyes are particularly affected; the muscles that control the eyes become weak, paralyzed, or uncontrollable in conditions called ophthalmoparesis… …However, respiratory failure is the most common cause of death in people with Leigh syndrome. Other neurological symptoms include peripheral neuropathy, loss of sensation in extremities caused by damage to the peripheral nervous system.”
I estimate these bad attacks to go hand in hand with deep hypoxia spread throughout much of the body. Sure, the lack of oxygen itself could do much of this ravage, but it also could “draw out” all thiamine for use in this “emergency relief ATP carrier”.
In younger Leigh patients, it also goes hand in hand with
“Infants with the syndrome have symptoms that include diarrhea, vomiting, and dysphagia (trouble swallowing or sucking), leading to a failure to thrive.[1] Children with early Leigh disease also may appear irritable and cry much more than healthy babies. Seizures are often seen. Excess lactate may be seen in the urine, cerebrospinal fluid, and blood of a person with Leigh syndrome.”
That once more has some overlap with “day to day” ME/…
I had thought of Leigh Syndrome in the past as so many of my symptoms connect to that. But my 23&me checked for the Canadian/French version and I wasn’t positive there. However, that is only one possible gene to check. Doing another search in another genetic site i belong to, show that Complement Factors dysfunctions can also lead to Leigh Disease. Th
I hit wrong button and wasn’t finished …..
The website hasn’t completed the gene searches to look into these Complement Factors to sort which genes connect to this dysfunction of what they are calling Leigh Disease by different Complement Factors.
But, I had a study of my Complement Factors and they are all 5 Dysfunctional and not working properly. So this could be a strong possibility here.
I knew B1 was helping my muscle weakness and energy. And kept asking Dejurgen to try it too.
Now, Dejurgen may have the WHY! to it helping!
I’m glad I didn’t let go of this one and kept “bugging” him on it.
I’m thankful for this blog and us all looking deeper into it. We may have some monumental discoveries here!
Thanks Dejurgen for keeping on digging for us!!!!!!!
And thanks Jeffrey’s for this blog and determination of it helping.
And to Cort, for letting us all bring our hypothesis to his Blog site and letting us explore them to find us ALL some more “Purple Bandaids?”!
Dejurgen asked me to mention again the reason I started trying B1 again (different form), as I mentioned it above. I had severe muscle weakness and inability to rise from a chair and also one eye started crossing.
If you look up symptoms of Leigh disease the version with Mitrochondria dysfunction connected, many of our symptoms fits the symptom list. And @Nancy B, swallowing issues are also connected to this.
My mom had swallowing issues and one of her eyes started to cross before she died. I wonder would B1 had helped her have a better quality of life too. It sure has helped me.
I’m not sure that its Leigh Disease by Mitrochondria cause, I know I don’t have the gene for the French version of it. But the information says when its genetic caused, it is passed by the mother. Can others think of family genetics and see is there a seeming link?
The cocktail given for Leigh Disease has B1 in it and also many of the things I was told to try for my Mitrochondria dysfunctions. The treatment for this disease is a mixture of supplements. (To note: some were way too energizing for me and I could not stick with them. It may not be necessary to throw the kitchen sink at things. But maybe one well placed missing component. And for me, less is MORE. I’m trying to get my body to come back on line to work optimally, if possible. Sometimes it doesn’t take a whole lot of something to do that. But a little may be enough. When I’m having a really weak day, I have boosted up to 200mg. But that is a rare thing for me to do. I find 100mg. to be where I feel best and more level.)
ME has IMO a tendency to be able to mimic other diseases. I guess that can be due to things like for example lack of energy production. A smaller underlying problem then IMO can sometimes present like the real diseasse.
In this case: not having the genes to for example make the enzyme needed for this thiamine diphosphate to thiamine triphosphate interconversion could cause this potential “emergency energy distribution system” to fall short. Having the genes working or only having some minor defects on these genes but having a very high demand (by having frequent systemic hypoxia) on this potential “emergency energy distribution system” could make it fall short too and hence represent with similar symptoms.
Issie having started to have her eyes crossing and having the eyes crossing being a symptom fairly typical for few diseases but Leighs syndrom and it stopping rapidely after high dose B1 intake may well point to similar up to identical mechanisms at work. So this remark about her eyes crossing might be right into the bullseye.
When I had a Mitochondrial membrane TL protein studies from Acumen, the Mt-membrane reported as very high for Aldehydes.
In addition Nitrosopyrrolidine, large protein complexes and a low intracellular pH were found under the section. “Chemical on TL sites”
I realise that theses tests are controversial but found the aldehyde connection of interest.
I want to thank you for this wonderful article – and thank the commenters for sharing so openly and helpfully.
I have hEDS/POTS/MCAS and suffer from intracranial hypertension symptomatically, primarily during low pressure weather system events. Long duration of these events are especially debilitating for me. I take Diamox symptomatically and it helps regain my brain and vision function until the pressure rises again. I’m keenly interested in seeing if Thiamine HCL could be an adequate replacement for Diamox. I did take benfotiamine in the distant past but can’t remember why I started taking it or why I stopped taking it.
In addition, I used Dr. Driscoll’s supplement, ParasymPlus for about 6 months. It dramatically helped my gut motility and energy levels and brain fog. The key ingredients are Thiamine, ALCAR, Choline, and huperzia. This is not an affiliate link, just sharing it here in case it helps/resonates with someone: https://secure.vagusnervesupport.com/products/parasym-plus%E2%84%A2
I stopped using it because I felt so much better and I was also watching my budget, so spending $60/month for one supplement started feeling a bit burdensome. I am already taking a lot of supplements, as part of The Cusack Protocol for EDS which has been a miracle for me – Highly Recommend!
In addition, I was experiencing extreme hair loss. I mean like fistfuls of hair was falling out. When I researched causes for hair loss, I came upon a reference to taking too much Thiamine. ParasymPlus only has 60mg, but I stopped it anyway. The hair loss continued for several months after that but has finally normalized.
Instead, I bought Bulk Supplements ALCAR and Choline/huperzia separately to attempt my own less expensive mix of her formula. But I felt so good on ALCAR alone, that I’ve not used the other supplements. My gut motility isn’t as good now that I’m off ParasymPlus, but my daily magnesium supplement seems to make me regular enough.
At any rate, I’ve shared this article in my local EDS group and I’m looking forward to trying Thiamine to help with my intracranial pressure, thank you!
@Christine, I looked up that protocol for EDS as I have EDS too. Arginine is known to cause issues with activating some virus and also increases NO. For some of us with possible low lying virus and maybe not needing more NO (vasodilates and some POTS people don’t need that…..I do however. But have to be very careful how much.) Some of the supplements could contribute to more problems if you have other underlying issues. For some with EBV arginine could activate that more.
@issie I am only taking the aloe, pqq, lgg, glucosamine/chondroitin, and hyaluronic acid of The Cusack Protocol. I do have EBV – but taking Low Dose Naltrexone (LDN) has helped dampen it’s activation. I did try the arginine at one point but stopped it (I can’t remember the reason). I did read about taking lysine on the opposite end of the day from arginine, but I didn’t do that. I’ve tried most of the other supplements on the protocol as well, but tapered back off of the ones that I could not tell were making a difference.
In Oct-Dec 2020 I did a supervised gut cleanse with a functional Dr., and it cleared up what was likely a type of SIBO. Since then, I’ve been feeling progressively better. I think that my improved gut function has enabled me to metabolize The Cusack Protocol supplements better.
There is a very supportive Facebook Group for The Cusack Protocol. Since it’s an open-sourced protocol, volunteers in that group help people as they start on the different supplements.
@Christine, good you are paying close attention to what things do to you and tapering down when you come off something. Good to hear your gut function is better and no more SIBO!
I just had a bad experience with a supplement that apparently activated Staph, it seems. And I have yet to recover. So why I felt it important to point out that citrilline, arginine and ornithine……may not be so good if there are other underlying issues. As some things feed the nasties and thats worse than the benefit they might could give. But if there is a broken cycle and there are no other issues…..I know they can sure help some people greatly.
But as I said below, we need to be aware of what we do and possible consequences. I was aware, but the tiny amount I tried, it was shocking to have such a big reaction. Why I say less is more and always go LOW and SLOW.
I did find that arginine helps (not activates it, mistyped above) EBV and some use lysine with it. Just being careful how much a POTS person vasodilates would be important.
I’m replying here as Issie is mixing things up and is too exhausted to reply correctly. She has been too active with her mind and overstepped her daily envelope.
It is mainly Staphylococcus and some other bacteria that can grow and multiply very rapidely on arginine (and citruline) supplementation. There are several research papers saying this.
Take for example the paper titled “Staphylococcus aureus ArcR Controls Expression of the Arginine Deiminase Operon” by “Julia Makhlin… …Yair Aharonowitz” saying
“In the absence of oxygen, S. aureus can grow by fermentation of glucose or by using an alternative terminal electron acceptor such as nitrate”
“resulting in the conversion of arginine into ornithine, ammonia, and CO2, with the concomitant production of 1 mol of ATP per mol of arginine consumed.”
“thereby enabling anaerobic growth in the presence of arginine.”
In short: Staphylococcus can grow in an environment with very low oxygen when enough arginine is present or supplied. Anaerobic growth often is fast growth, much like anaerobic convertion of glucose to pyruvate goes rapidely. We don’t want Staphylococcus provided with high amounts of anything that can dramatically boost their growth, especially knowing that several Staphylococcus species are resistant to near all antibiotics.
So Issie’s experience and advise: be carefull when supplementing either arginine or citruline. Even if some helps, plenty might not be better.
Okay, I’m a little more rested now.
I meant to type it could activate Staph and strep and some other nasties. I felt it important to note this.
I am still recovering from an infection that may had been contributed to by one of the supplements he spoke of. I’m no longer trialing it. As I’m still trying to get over the end results. (And the amount I took was a very, teeny, tiny amount. So even a small amount can trigger an underlying, laying dormant, problem and wake it up……it seems.)
I asked Dejurgen to correct it for me, as he said….. I went past my limit with research and commenting and was starting to type in wrong words in my comments.
Thanks Dejurgen, for helping me yet again! Okay, done for today!!!!
glad so many here are willing to share and make such tremendous team; so heartening
(Looking forward to your book Issie and dejurgen—maybe it will be downloadable for a fee?)
hypothetically, could supplementing stop liver problems for people with NFLD?
If we ever get to writting down large chuncks of our info, then we plan to write it on HR, for fRee. The plan is to get this year a sizeable chunk out, but the task is rather daunting as we want to make it both easier to understand yet better embedded in science.
As to NFLD, Issie and I have found quite a bit of info pointing towards hypoxia or similar oxygenation problems being able to create problems of converting carbs and proteins to fat including in the liver at high rate. So according to those ideas things that can improve oxygenation should be able to help *some* to reduce the NFLD problem.
As written above, we both think it’s better to go low and slow (think from zero to final dose in one year, yes year) with anything that can improve breathing a lot as the risk for strong reperfusion like injury is real.
I tried this years (decades) ago when fat-soluble forms of thiamine were not available in the U.S. and you still had to get benfotiamine overseas, from places like Germany. Needless to say, it didn’t work. As an energy co-factor it can supply some energy if you’re deficient, but lacking that I don’t think it is going to be any miracle cure for ME/CFS.
One thing to be careful of is that excessively high doses of thiamine over time can downregulate your own thiamine systems, leading to nasty deficiency syndromes like Wernicke’s encephalopathy if the external thiamine supplementation is suddenly stopped or reduced. (Don’t ask me how I know that.) In some cases the syndrome can be reversed in full or in part by reintroducing the thiamine, with perhaps even higher doses than initially taken, and then very, very gradually tapered only after a good period of stable recovery (could be weeks or even months). If not treated immediately, it could lead to permanent neurologic damage and even death. Caveat emptor whenever taking supra-physiologic doses of ANY supplement.
I looked up Weirnickes encephalopathy and it is said it can be gotten by too much alcohol ingestion and it depleting B1. The treatment is high IV treatment with B1 and then indefinitely continued oral treatment with B1. This illness can be fatal if B1 is not supplied. It can also bring on a form of dementia and ataxia. Ataxia is having issues with walking and strength.
I’m going to tie this in with what Dejurgen and I have been talking about with issues with acetaldehyde and it going too high…..despite NOT drinking alcohol. This can happen by things within the body, like pathogens, yeast. But, can also happen by certain body processes that go wonky. It is possible that this dysfunction with acetaldehyde could possibly cause this type presentation of low B1 that this illness speaks of.
B1 would be used to stop the presentation of this disease. I don’t see that it would cause the illness. (Please correct me if I’m missing seeing that.)
If you are already demonstrating signs of this illness and are not ingesting alcohol to cause it, then what could be the cause of it? Dejurgen and I, and a few others, hypothesis too high acetaldehyde, as one piece of the puzzle. We are working on ways to get that down. But if there are obvious signs of a lack of B1…..this might be a good bandaid.
I agree that Benforthiamin didn’t work for me either. But B1 HCL, does make a difference for me. And, I’ll also say, it was needed despite my already working to lower acetaldehyde. So it was an additional need for me. (And, another caution…..we said above……don’t lower acetaldehyde too much. It can lower dopamine too much if you do. And that is not good nor fun. That was a hard learned thing, by me.)
I do however try to go the lowest I can to get results. I don’t take massive anything. I’m trying to bring my body back into homeostasis – My desired end result. And hopefully with more balance, eventually supplements won’t be necessary. I’d much prefer to do it with diet. I do believe that any concentrated supplement is more like a medicine. And keep in mind too much could imbalance something else. And the body has to process it. So with too much, it could create more stress, not less.
Forgot link on that disease that needs B1 to correct it.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5354137/
BTW, B1 can so lower acetaldehyde.
Alcohol itself doesn’t cause Wernicke’s, excessive alcohol consumption depletes B1 which can then cause Wernicke’s when carbohydrates are added. Anything that depletes B1 can therefore lead to Wernicke’s, including downregulating your B1 enzymes by taking high doses of B1 and then stopping or reducing B1 without tapering it. The high B1 dosing creates a functional thiamin deficiency through enzyme downregulation, which then requires those same excessively high B1 amounts to maintain functional co-factor levels. If you stop the B1 suddenly, or reduce too much too fast, you are at high risk of Wernicke’s because of the functional deficiency you’ve created by the excessive dosing. You need to maintain or raise B1 levels to reverse the Wernicke’s if it has manifested, and then a gradual taper to restore your enzyme function through upregulation once you are stable. If you haven’t died, of course.
@Rick, that makes perfect sense. And why I try to go as low as possible with any supplements. I think its better to do things with diet if it at all possible. But with mh supplements, I cycle and also taper.
I consider supplements concentrated and powerful. And at that concentration, becoming a medicine. If someone doesn’t know what they are doing, they can for sure mess themselves up. Not knowing well what processes/pathways things work on and there being something else going on, that could be the wrong thing to do with certain supplements/herbs (especially).
I take thiamine HCL but I find it works much better with magnesium Glycinate and r5p, niacinamidle, calcium pantothenate, p5p, and biotin. Took me a months to find the correct doasges for each by emptying out each capsule or each b vitamin. And I take folate and b12 separately.
This combination seems to help temporarily bring back feeling and some more strength to my muscles and reduces my air hunger or helps when my body crashed into what I call an atp crisis where I cannot move and my brain and cells feel lack of oxygen no matter how much I try and breathe.
Late I tried thiamine cocarboxylase under upper lip by itself and it was a dramatic effect in my muscle strength but after a while it would put me in the atp crisis so had to stop taking it. But it was very dramatic. Which I knew why it did that.
I can follow both of you:
“leading to nasty deficiency syndromes… …if the external thiamine supplementation is suddenly stopped or reduced.”
“So with too much, it could create more stress, not less.”
Unless there is a *constant* downregulation of B1 transporters, adding massive doses will disturb the balance in several fairly important biochemical processes. I don’t estimate that the majority of patients (gut feeling) that improves significantly with high dose B1 has such constant downregulation of B1 transporters.
If so, IMO (Issie and I not fully agree on this one yet) high dose B1 supplementation has potential high side effects that are offset by even higher beneficial effects (in those that receive clear benefits). If that were true, just switching on and off mega dosing B1 could potentially indeed exaherbate side effects during abrupt transitions in some patients.
Also, if that were true then there may be potential to get better results with high dose B1 by better understanding what it does and better recognize and compensate potential disturbed processes.
Thank you! There is an alternative explanation for the possible effect of thiamine that I´d like to refer to:
Thiamine is a central cofactor for alpha ketoglutarate dehydrogenase (KGDH), a key enzyme involved in mitochondrial energy metabolism.
It can be shown that KGDH is severly inhibited in neuroinflammation – possibly related to glutamate neuroexcitotoxicity (https://www.sciencedirect.com/science/article/abs/pii/S0891584917308912). Indeed, the application of high dose thiamine has been shown to reverse the effects of glutamate excitotoxicity (same reference).
This may also explain why in an animal model high dose thiamine supplementation can preserve mitochondrial function after traumatic brain injury (https://pubmed.ncbi.nlm.nih.gov/29777685/).
So possibly high dose thiamine may have neuroprotective effects by dampening neuroinflammation.
Indeed, the elevation of alpha-ketoglutarate seen in a metabolomics study of ME/CFS (https://www.mdpi.com/2218-1989/8/4/90/htm) may possibly be a reflection of KGDH inhibition in this disease, too.
For details on this hypothesis see a summary at https://www.eonutrition.co.uk/post/mega-dose-thiamine-beyond-addressing-deficiency
@Herbert, thanks for your links. Too high glutamate is another one of the puzzle pieces we are also working on. Nice references and for sure could help. Too much glutamate has been a hypothesis of mine in connection to POTS for over a decade now. And it does seem to apply to ME/CFS too!
Thanks for sharing Overton’s hypothesis. Very interesting. I will take a closer look. One broader point is that multiple people in different countries are observing that high-dose thiamine leads to benefits in people with no thiamine deficiency.
I do 100mg thiamine injections, it get reduced symptoms for like 3 days to a week after the injection. So twice a week is the sweet spot for me.
Thiamine injections are also used in the case of sepsis chock, makes you wonder if bacterial translocation through the guts plays a role or thiamine has some effect we don’t know yet on the immune system.
Though I’m betting on the PDH up regulation, since PDHK is elevated in CFS.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7024754/#:~:text=In%20one%20study%2C%20123%20patients,%2Dday%20mortality%20(32).
I looked up about thiamine and septic shock. I didn’t know it was given for this. Here is a good article describing what it does in that case. Appears they also use Vit C with it. Appears that it helps bring Lactate levels down.
There is hypothesis that we may be in a state of Chronic Sepsis.
Took 600mg B1 and the following AM had a nose bleed with blood in urine per reagent dip stick.
Two days later did a repeat with the same results. It appears to thin my blood but that effect I can’t find in literature. No other changes in symptoms.
Several others reported Mmega dose B1 can provoke MCAS like effects. That is a fairly strong immune effect and should be able to thin barriers.
My frequent nose bleeds seem to (not certain) coincide with either dry air (high confidence about this cause) and unmedicated (but doing things to lower sensitivity) hay fever (repeated observation, harder to check).
Thank you for reporting your experience, @RonP. The Costantini team has treated more than 2,500 people with high-dose thiamine and does not report this side effect. However, nose bleeds is listed as a rare side effect of acetazolamide, another carbonic anhydrase inhibitor for which there is a larger base of experience. Acetazolamide increases blood flow to the brain, potentially due to the production of carbon dioxide, so it can certainly have effects on the circulatory system. This is one potential explanation for the benefits of carbonic anhydrase inhibitors, but I could see how this attribute might lead to problems in certain cases. I wonder whether individuals with certain vascular or circulatory issues are more prone to this issue?
One note for other readers — the literature reports a delay in the effects of oral high-dose thiamine HCL of 2 days or so. So if one started on day 1, and continued ongoing administration, you might expect to see the full effects only on day 3 or 4. My daughter uses divided doses 3 times a day.
I take thiamine HCL but I find it works much better with magnesium Glycinate and r5p, niacinamidle, calcium pantothenate, p5p, and biotin. Took me a months to find the correct doasges for each by emptying out each capsule or each b vitamin. And I take folate and b12 separately. Because both can tank my potassium levels
This combination seems to help temporarily bring back feeling and some more strength to my muscles and reduces my air hunger or helps when my body crashed into what I call an atp crisis where I cannot move and my brain and cells feel lack of oxygen no matter how much I try and breathe.
Late I tried thiamine cocarboxylase under upper lip by itself and it was a dramatic effect in my muscle strength but after a while it would put me in the atp crisis so had to stop taking it. But it was very dramatic. Wish I knew why it did that.
B9 and B12 pulled me out of the ME/CFS sinkhole in the nick of time, just as I was becoming severily-severily ill. B1 then got me out of bed.
It was tricky at first: I found that both Hcl and benfotiamine made symptoms much worse (including peripheral neuropathy, which supposedly benfo is the best at ameliorating…) – TPP has been my magic pill. I take small doses through out the day and this seems to work for me.
I also went though the… adjustment period – for me it lasted a few months. Pretty screwy at first… Anyone who is interested in taking B1 benefits from reading through the hormones matter website first and Elliot’s posts/videos too. To start slowly, to titrate slowly. Magnesium and other Bs are recommended – though one should watch out because a large group of people cannot handle B6. For this reason, I prefer taking the Bs individually at first. I don’t do well with B3 – perhaps it is as with the B1, and it is a matter of finding the vitamer that works for me. B12 I also had to play with the different available forms. I’m keen on sublingual, transdermal or liquid forms of nutrients.
I view B1 as a gift – the opportunity to get a leg up on correcting metabolism. It is crucial at the same time to work at restoring metabolic/thyroid function and reducing oxidative stress and lipid peroxidation. Otherwise any of the supplements are simply a bucket removing water from a drowning boat. This is done through how one eats, pacing, increasing CO2, etc , and movement when one is ready. Our bodies are mining muscle AND connective tissue for proteins, whether one has hypermobility or not. The ‘right’ physical rehabilitation to work at correcting faulty muscle patterning and joint instability. There is a view that the ‘right’ kind of exercise promotes mitochondria biogenesis. And also, the ‘right’ kind of exercise stimulates collagen production and strengthens it.
Indeed, reducing anaerobic metabolism can reduce lactate production and protein consumption. When we can reduce *the need for* anaerobic mechanism enough, we could reduce the need for the emergency use of our muscles and connective tissue as a source of protein. I wrote about this in the first comment of https://www.healthrising.org/blog/2021/03/30/muscle-fibromyalgia-chronic-fatigue-syndrome/ and further linked it to Issies ideas of glutamate excitotoxicity.
If B1 can reduce anaerobic metabolism by shifting towards aerobic energy production, it can reduce damage building up to our muscles and connective tissues. Other ways to reduce it are described in the comments on that blog.
Connections between muscle and connective tissue destruction and unstable blood sugar are described. How this links to children and men being less vulnerable to ME/FM/EDS is explained there as them having more growth hormone and testosterone respectively, helping them to rebuild muscle and connective tissue faster. In other comments below it is explained how this hypothetical mechanism can help explain post viral illness even if all virus is eradicated from the body.
In such, it also explains the need to rest loooooong enough after all virus is eradicated from the body in order to not trigger a vicious circle where the dire state of our muscles and connective tissue (impairing blood flow and increasing inflammation even at rest) triggers a new round of muscle and connective tissue pillaging upon *both* physical and mental exertion. The latter may seem strange, but high effort long lasting mental activity can exhaust the aerobic envelope and may need to delve into the muscle reserves for protein too.
Meirav, this is wonderful news! I just interviewed Dr. Lonsdale (who is 90 years old!!) for a Long Covid and ME/CFS summit I’m hosting and have been looking to interview someone who has ME/CFS/FM and has benefitted from high dose thiamine therapy. I’m not sure what the protocol is here and don’t want to step on any toes, but I’d love to see if you’re interested? If not I totally understand! If anyone else would be interested…
I, myself, have been trying out Lipothiamine (TTFD form of thiamine) and it was too much for me…I’ve backed down and will start again with thiamine HCl with appropriate cofactors and other B vitamins to balance.
α-ketoglutarate dehydrogenase is another enzyme that is inhibited in Kreb’s – and thiamin supps also gets it going. I get the sense that pyruvate carboxylase also could be inhibited (biotin may help some, tricky for others). TPP is also a co-factor for transketolase, which precedes production of metabolites used in the production of sphingolipids and phospholipids.
The view of bioenergetic and cell physiology dysfunction underlaying diseases was first postulated decades ago – Dr Lonsdale himself has written about meeting with Hans Selye back in the days.
https://tinyurl.com/et36phh5
Cell Danger Theory to me sounds at its core = the General Adaptation Syndrome. Whether one subscribes to the model of plasma membranes or not – both the work in that field and Naviaux observe the same shifting of ATP and minerals from inside to outside the cell. I like to think that Naviaux and his team are furthering knowledge in this field: they noted the movement of nutrients – B1, B3, B6.
https://tinyurl.com/twux9f2h
I read a recent Chronic Kidney Disease metabolomics – the findings were strikingly similar to the ones made by McGregor/Armstrong’s team in ME/CFS. Meaning that the adaptations for energy production are similar, meaning that mitochondria dysfunction underlays more than one disease process.
And there is someone who has been writing about this for decades as well, combining the work of all these scientists into a big picture of how different systems interact in disease and in health
https://tinyurl.com/2wzk6vzc
If one finds it hard to wraps one’s head around the idea that the basic principles of biology may be different from what is thought, maybe this can warm one up it:
“Is the standard model broken? Physicists cheer major muon result”
https://tinyurl.com/teukyv96
And it’s not the first time thiamin has been written about in Health Rising! Over the years, this platform has offered the bits and pieces that make up the polypill to treating ME/CFS and FMS.
“Pure T3 Thyroid and Stories of Recovery from Chronic Fatigue Syndrome (ME/CFS) and Fibromyalgia: An Overview.”
https://tinyurl.com/wkrajavy
“Hypothesis: Mechanisms That Prevent Recovery in Prolonged ICU Patients Also Underlie Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)”
https://tinyurl.com/mvsnhzpc
I long for Dr. Lonsdale’s work and ideas to be noticed by the ME/CFS community, and the medical world at large. May this bring more health to many.
{you’ll need to add the www to the links, I think my comment wasn’t going through when I was including full address}
Looking forward to following your results, and any protocols that result. As I told you, I used thiamine (including benfotiamine) for a number of years, and thought it helped, but didn’t notice much of a change when I weaned off it. I’ve tried it for a couple of weeks twice since, but didn’t notice anything dramatic like some people report.
I don’t have any medical people involved that I would go to for an answer, and am semi-functional (I write fiction) for a period every day – I would love to improve that, but also find that it takes way too much time to try to figure things out on my own.
Good luck with your research, and I look forward to hearing more, especially if a test can show how much I need, and I need more than I have. My ability and interest in playing around with the thousands of things that people claim help them is not large. If something has a real effect, I hope it will eventually become well known.
Is taking thiamin nitrate the same as thiamine HCL ? And what do the acronyms “DAO” and “MCAS” mean please
MCAS is Mast Cell Activation Syndrome, where there is an over response of the body with mast cells degranulating. It is not really due to a true allergy, but can lead to anaphylaxis.
DAO, is a type of enzyme that our body makes, and can be deficient, to properly break down histamine.
Thanks Issie,
Is taking thiamin nitrate the same as thiamine HCL ?
Its chemical structure is different. Thiamine HCl is a form of thiamine (vitamin B1) that has been bonded with hydrochloride (HCl) to improve absorption. Its more soluble in water.
Some have mentioned other forms that have either worked or not. This HCL is the form that seems to work for me. I haven’t tried the cocarboxylase form. What I’m finding is that form is a lot lower strength. Some are saying that form is working well for them. So maybe trying different forms, we will find our best fit.
https://www.healthrising.org/blog/2021/04/13/muscle-pressure-fibromyalgia-old-paradigm/
In Dejurgen first comment on this blog he talked about in anaerobic metabolism the body uses lactate and protein. If we can reduce the use of both, it can reduce the need to use the muscles and connective tissue. He also talked about when the immune system is in strong activation it uses plenty of protein and glucose that could be pulled from the muscles.
If B1 can shift the anaerobic metabolism to aerobic energy production it can reduce damage building up in our muscles and connective tissue.
Lots of good information that Dejurgen talked about in this blog.
Thanks Issie for your help
I’m a member of Genetic Lifehacks and I just got an email from Debbie Moon, writer and owner of that Blog Genetic site. She gave me a link on genes with B1 issues. I’ll also post what she said as these are not the only genes to look at. And checking mine, 23&me didn’t do a lot of them.
I have an article with several (rare) genetic mutations that cause an increased need for thiamine;
https://www.geneticlifehacks.com/thiamine-genetic-variations-in-need-for-b1/
But, there are more mutations than just what is covered in 23andMe/AncestryDNA data, so you can’t really rule anything out if you don’t carry those specific mutations. I just set the thiamine article to be ‘public’ instead of member’s only, so if anyone who is benefiting from thiamine supplementation on health rising wants to check for thiamine mutations they can do so without needing to pay to join genetic lifehacks.
Hi Issie….Thanks so much for all your input. I have benefited from HD Thiamine and want to explore the idea of genetic testing. My question is; as I have found something that works, will it be of benefit to know if I have a genetic fault or will knowing not change what I can do to overcome it. I have already got children so too late to think of that! Fergus
I have been taking florinef (fludrocortisone) 200mg daily and just added b1 hcl at 600mg daily. Nothing has changed with my pem and I’ve been stuck in bed for 4 months. Also on b12 100mcg daily and a whole bunch of other supplements. Any idea if florinef is impacted by other medications or supplements?
Hi @Dom, I do not know if florinef is impacted by other medications. I will say, however, that florinef appears to have made my daughter (EDS/ME/CFS) worse, triggering heightened mast cell issues. I know it has helped a lot of people, but I am concerned that it can exacerbate intracranial hypertension and ventral brainstem compression among people with ME/CFS or EDS who have cervical instability and/or chiari malformation. One of my long-term projects is to see if there’s a way to determine who benefits and who is harmed. Have you had a good experience?
Have you had your daughter tested for Lyme disease? My husband’s ME/CFS was Lyme induced. Fortunately I found a Functional MD who himself had the same ME/CFS symptoms, did proper testing for Lyme and coinfections through Armin labs. Once LYME was treated he recovered.. My husband is having the same good results. He’s on a new treatment for Lyme with a repurposed drug called Disulfiram. His symptoms are improving. Rule out Chronic Lyme disease in your daughter through a good Lyme doc and test. All the best.
Hi, I have been using Bemfothiamine 300 mg for nearly 3 months. Excelrnt results: more energy, less or almost no neuropathic pain, less intestinal peoblems ( I have Fobromyalgia and IBS. What so you advice? How long time can I take Benfothiamine? Shall I make 1 month break after 3 months? Thanks!
Glad to hear you have benefitted from high-dose thiamine! I cannot provide medical advice, but I will say that my daughter has been taking high-dose thiamine now for 17 months without a break and continues to experience benefits. I have put together some cautions that we follow to promote safe long-term use: https://www.edsperspectives.com/cautions . These are premised on the assumption that high-dose thiamine operates as a carbonic anhydrase inhibitor in people, just as it does in in vitro studies. As always, recommend consulting a medical professional for advice. Good luck!
Can I start direct om high dosage of Thiamine, or do I have to go slow up?
Hi Anne, I am not a doctor and cannot provide medical advice. I will say that when my daughter started, we titrated up slowly over the course of several weeks. This is our general approach to most supplements. It can take 2 or 3 days before the effects of an increase in high-dose thiamine are felt.
Thank you for this interesting post @Jeffrey! I wrote this in a response to someone else, but you might be interested in the interview I just did with Dr. Derrick Lonsdale for a summit I’m hosting on Long Covid and ME/CFS. Dr. Lonsdale co-authored the book Thiamine Deficiency Disease, Dysautonomia, and High Calorie Malnutrition and is a main contributor on the site http://www.hormonesmatter.com that has tons of information on high dose thiamine. He’s been using high dose thiamine for many decades (he’s 90 years old!). The interview will be released the week of July 5-12.
I’d love to take part in your survey but think it might be best to wait several months. I started high dose Lipothiamine over two months ago and recently had to stop because I was having very bad reactions, which I believe are either what Dr. Lonsdale called “paradox” and/or are from imbalances in other nutrients, cofactors, etc. TTFD can cause more imbalances because it takes more to process it into useable thiamine, which makes it more bio-available, but also means it can cause more problems than other forms.
Let me know if you’d still like me to take part in the survey, I’m happy to.
Some of you have mentioned flushing in these posts as a reaction and this is a new symptom for me. It feels like an extreme allergic reaction, my face burning and visible red splotches, also feels like my face is swelling up. It’s painful and scary. Benadryl plus 800 mg ibuprofen relieved it, slightly at first, completely within about 40 minutes. So does this sound familiar? It’s happened twice in the last few weeks, and has never happened before. I have started some new supplements so I suspect the culprit is there. Could be foods; i think giving up nuts helped with some of it, but not all.
The other thing is all kinds of skin problems that have kept getting worse for the last 10 or 15 years. Started with ezcema, then the hives started (huge welts on my back just from taking a hot shower). Waking up with deep scratches across my back (itching in my sleep). I’m all scarred up at this point. Then the rosacea started a couple years ago. So that’s fun.
Does this sound like MCAS?
Could it be exposure to toxic mold? (I test high for mold in my body including the ones that make mycotoxins.) Also candida. Dr putting me on liposomal glutathione to help detox. So whatever is happening is also accelerating. Any experiences with this appreciated. I’m sure there is a thread somewhere else about this, I need t check.
Hello, wonderful article. Might I ask what brand Thiamax is best, and should magnesium be taken in conjunction with this?
Thanks. The need for magnesium would depend on which hypothesis is correct about why high-dose thiamine works. If it is through carbonic anhydrase isoenzymes, I would not think it is needed, but if it is through passive transport of thiamine, magnesium could potentially be helpful. For what is worth, my daughter takes it as part of her supplements. We use Thiamine HCL (GNC brand, 300 mg tablets) and don’t have personal experience with Thiamax.
I’m literally wanting to start Thiamine HCL, a B complex and magnesium immediately. I’ve had brain fog for several weeks, but the worst thing is it’s like my GI has slammed to a halt. I have no appetite, terrible constipation, bloated, belching, along with sleep issues and tinnitus. I think my IBS-C from all these years it’s either B1 deficiency or the IBS has caused a deficiency. Chicken and egg. I’ve done all the hcl, enzymes, fiber, etc etc etc.
Either way, I’m at this moment.
I’m want to try b1 hcl, b complex and magnesium.
Why would I not just try 3x day, 500 mg of the thiamine hcl and those other supplements? Can I do that day one, spread out?
Thank you all for all the information. I believe I understood about 25%, maybe. As I was reading I was thinking about my daughter who has Wernekes encephalopathy from alcoholism. I knew the treatment was high doses of vitamin B1. She has recovered use of her muscle function. But ended up with permanent memory damage. She has short term memory problems. She does not remember what she did the day before unless reminded with a cue. Interesting that it was mentioned as I read all the comments. I was wondering about the relationship as I read along because I knew it helped her. This is an article I had saved but not read yet. I had read Jeffreys later article on mega doses of B1 for helping fibromyalgia. It was enough to go out a get a bottle of B1 and give it a whirl. I started with 300 mg then moved up to 600 mg taken in the morning and mid afternoon. I did notice significant energy improvement within a couple of days. Had a bit of get up and get things done energy. Have just been taking for 2 weeks. Have already noticed the effects seem to be wearing off. That is what usually happens with me. I do well for a short time then it fades away to zero. I know that is a problem for many of us. Thanks again for all the information and links.
Hi @Martrecia, Thanks for sharing your experience. Sorry to hear the effects may be wearing off. I do not know why this happens for some people. I cannot speak to your situation obviously or provide medical advice, but here are some possibilities I’ve thought of for this issue generally. One possibility is that the effect wears off as another necessary nutrient is depleted. Magnesium is a possibility as studies have found that thiamine supplementation for Wernicke Korsakoff syndrome doesnt work in people who have low magnesium. The remedy would be magnesium supplementation. Another possibility is that some people feel a lot better on thiamine and then overdo it, and break through their aerobic threshold. and crash. The remedy here would be to continue pacing and only slowly increase physical activity. If thiamine wears off over a longer period of time it may be because of acidosis but i would be surprised if this happened so quickly.
Hello I recently purchased Mega Benfotiamine at 250 MG. . The label indicates it is both water and fat soluble. (is that ok?) I began my B1 titrate two weeks ago and have been slowly building up to 1500 MG a day. I might try to push it to 2000MG. I have not noticed any results thus far but I am recovering from a cold. Hopefully I will see some marked improvement in the next week. I have dealing with CFS for over 10 years now. For some reason lately my symptoms are getting wore. I have a scheduled MD appointment to discuss trying the low dose Abilify .
While there are a few reports of people only benefitting at very high doses, in general, I would expect that someone who would benefit from high-dose thiamine to be experiencing benefits already at 1,500 mg daily (and indeed at lower doses as well). As reflected in my write-up of the survey results (https://www.healthrising.org/blog/2021/06/02/fibromyalgia-chronic-fatigue-syndrome-benefit-high-dose-thiamine/ ) a number of people report no benefit from high-dose thiamine. While the share of non-respondents overall is small, it is higher for some subgroups. In particular, about 40% of people with ME/CFS and POTS but not EDS or Fibromyalgia reported that high-dose thiamine did not work for them. It appears they may need a different treatment approach. Some people who did not respond to thiamine HCL or benfotiamine report benefitting from TTFD (also known as lipothiamine or allithimine). That crosses the blood/brain barrier and is generally taken in much smaller doses (50 to 100 mg daily). Some people report feeling worse initially on this but then feeling better after what Derrick Lonsdale calls the “paradox” passes. I don’t have any direct experience with alternative forms of thiamine like TTFD but you could look up Londsdale’s writing on it.
I was diagnosed 3 years ago at age 56. Symptoms were tremor in right leg, loss of handwriting ability, and soft voice. I also have difficulty rising from a seated position and have balance issues. I started out taking only Azilect, then Mirapex, and 6 months ago Sinemet. Several months ago I started falling frequently, hence the reason for Sinemet. I tried every shots available but nothing worked. I have been reading about thiamine and want to start him on high dose therapy (hoping that his symptoms do not become worse). In June 2018, my neurologist and I decided to go with natural treatment and was introduced to Dr. Fabien, i contacted him at http://www.kunimeherbs.com. I wanted to know more about him before I go ahead with the kunime herbal medicine. I was extra cautious about anything like this. I had a few doubts in my mind, I have searched all over. he explained to me how the kunime medicine works to cure Parkinson’s disease. I discussed with my husband, he permitted me to try it. I made an order for the herbal medicine. I started using the medicine, I noticed tremendous changes, I had a total decline of symptoms with this treatment, the Tremor, falling frequently, stiffness, body weakness, balance issues, depression and others has subsided. After 5/6 months of using the Herbal medication, I was 100% free from Parkinson’s disease. I’ve really learned to appreciate life now. Having this disease has taught me patience and not to take the little things such as being able to talk or to smile for granted. This treatment is a breakthrough for all suffering from Parkinson’s, don’t give up Hope. Kunime herbs are truly gift from God. No more people have to be diagnosed and burdened with this disease. 409 209-0379
I beg to differ that Thiamine is in good supply in our modern diet. It’s actually vey lacking, especially in the processed & highly refined, nutrient-stripped refined wheat & sugar-laden ‘Carbage’ that makes up so much of our modern diet. This nasty pseudo-food actually uses up way more Thiamine than it supplies. Thiamine deficiency is rampant in our modern society.
Dr. Lonsdale’s book (Thiamine Deficiency, Dysautonomia & High Calorie Malnutrition’ very much explains this.
Also, taking Thiamine in isolation is not advisable. B vitamins are synergistic & all support each other in various ways. Taking one may potentially deplete others. I find if I increase Thiamine I also need to increase my active sublingual B12 or I get symptoms of low B12.
Hi there, I have ME/CSF and I am trying high-dose Thiamine as prescribed by my nutritionist – taken alongside support for the adrenals and thyroid. It has been around a week only, but I found that I reacted strongly within 24hrs – with a very high heart rate and difficulty sleeping. I have reduced and reduced again the dose, and am now trying 1/8 of the original prescription. It still leaves me wide awake until 1-2am, though my heart rate has normalised. I should mention that I take a low dose of Amitriptyline to help with sleep dysregulation (I struggle to get deep sleep) but the Thiamine seems to have cancelled the benefits out completely.
Has anyone else experienced a racing heartbeat and insomnia as a side-effect of taking Thiamine for ME/CFS?
For information on Thiamine, please check out the http://www.hormones matter.com website. Elliot Overton also has quite a few videos on YouTube too (EONutrition).
I tried thiamine a few months ago and it didn’t help. However, I was reacting to my second covid jab and things were quite bad at that time. I started again last week and I can certainly see a difference in my energy. One of the surprising differences is that I keep wanting to eat! I have researched that and I see that people who are deficient in thiamine end up eating less because their body reaches satiety sooner. Supplement with thiamine and the appetite comes back. I have known for some years that I eat much smaller portions than anyone else I know. This is interesting. However, I should not be deficient. I take a very good multi-vitamin that provides 40mg of thiamine per day. Perhaps the thiamine has all been used up for energy production or all the other stuff and there was just not enough to regulate appetite.
Also interesting is that my asthma (all allergic – only happens in Summer) has been out of control this Summer. I spoke to a doctor and got new medication and started on Saturday. However, before taking it I realised that the coughing had not been as bad on Friday. I have noticed today on a re-read that your article talks about thiamine being a histamine liberator. I wonder if the reduction in my symptoms this week is down to the thiamine and not to the new medication.
Lots of things to ponder!
Thank you for this blog. I’m glad I tried again.
For information on Thiamine, please check out the http://www.hormones matter.com website. Elliot Overton also has quite a few videos about it on YouTube too (EONutrition).
Why is Thiamax no longer available, anywhere? Is the manufacturer been “discouraged” from further production?
Wow, this page is near impossible to read! Too many comments, you probably won’t see this but here goes; a popular doctor said that B1 could improve many of the conditions I have (too many to name but ME/CFS & autism etc.) The problem is P-5-P makes me violent, as well as a host of other synthetic B’s like metafolinic and forgot which was the 3rd I tried. I have severe MCS and don’t do well with synthetics. The 1st pill of P-5-P made me very calm & almost normal, very rested. All subsequent make me violent. I don’t know if it has to do with malabsorption, gut problems or my impaired liver. I can barely function. I placed an order of Benfotiamine (the lowest dosage I could find) which also contains thiamine. I will take 1/3rd or 1/4th of the capsule to avoid problems.
Why can’t these vitamins be found in NORMAL food supplements? Why are all B1 bottles synthetic? I eat n. yeast but it doesn’t have therapeutic effects. I was taking “food-based” B’s but then found out that the mfrs add synthetics to them! (Plus G.O.L was sold not so long ago, so quit buying that. MF is the same: claim to be food-based until you read the fine print: they add synthetics to them!)
I don’t absorb much nutrition from the foods I eat and suffer from severe pyroluria. .Not much info out there on this but it’s causing me to have gaping cavities that won’t stop growing no matter what I do, I have huge holes in my mollars at the gum base. Any help for that? Thanks in adv, if you saw this and if I remember to find this page.
I cannot provide medical advice but am happy to share my personal experience and general understanding of the situation. P-5-P is an activated form of the vitamin B-6. This is a different vitamin from thiamine, which is vitamin B1. My daughter could not tolerate P-5-P. But she tolerates well a B complex vitamin that includes a balanced combination of different B vitamins, including Thiamine (B1) and a smaller amount of P-5-P. She takes the Thorne Basic B Complex. Respondents to my survey generally liked benfotiamine as a source of Thiamine (Vitamin B1). Following the advice of our doctor, we generally start all new medications with a small amount and work our way up slowly over time to higher doses. Currently my daughter takes high-dose thiamine hydrocholoride and a tiny dose (1/8 of a 50 mg pill) daily of allithiamine, a special variety that crosses the blood-brain barrier. Good luck!
Wow, thank you for your reply. Well I just took my first pill of Benfotiamine and horrible side effects! I have POTS and already dangerously low BP. It lowered it even worse!!! I am still so dizzy as I type. I first took only 1/2 a capsule but the bottle says 4 per day, so took the other half. Huge mistake. I took it with a fat balanced meal. The benfotiamine also has thiamine. I might try the Thorne, as I take their zinc & adrenal complex. I should have stuck with the half. I don’t know if I’ll try this again. maybe 1/8th and see. At least it did not make me rage, but this could make me lose my life. Glad that your daughter found something that works for her. I’ll stick to my yeast flakes for now. Again, thanks for your input!
Thiamine produces CO2? How? I’m very interested in this. I have an extremely low control pause of 0-1 (Buteyko method) which supposedly means my CO2 levels are very low.
I unfortunately developed cluster headaches from TTFD. I started with 25mg. Got terrible headaches, stopped, gave gap, reduced to 5 mg. Tried on and off over months and by that developed disturbances in my sleep/wake cycle which was the start of my clusters (I understood later). Thereafter got cluster headaches, took a year to get diagnosed. My life has been destroyed since i am suffering from cronic clusters now, am loosing everything and living in pain constantly. There is no doubt it’s TTFD, because I have worked with it enough to understand what is what.
Are you saying that the cluster headaches induced by ttfd persisted after stopping?
Have been taking thiamine 500-1500mg/day Qam on and off for decades since reading an article about it on Prohealth.com a long time ago. It has helped a lot with Chronic Mono/Fibromyalgia/Chronic Fatigue/Lyme/etc. diagnosis. I stopped taking it for awhile and developed IBS, which got better when I started taking it again.
Thanks for sharing your experience!
Upon finding this group of articles we decided to give a chance to high dose thiamine for her fibromyalgia with positive results. We started with 1 pill of 200mg and upped the dose 200mg every two days until we found the good spot where she started to feel better. We stopped at 600mg a day, 300 on the morning and 300 at night, and she hasn’t had an episode of intense pain as she used to have before these last couple of months. Particularly noteworthy that when she had other sicknesses or had little sleep she before had noticeable mental fog and a painful day, whereas she got a flu and several days where she had to sleep less than she should and she didn’t have the same amount of mental fog and pain episodes. She’s not entirely well still but she’s better by her own estimation. God bless Jeff Lubell, this website and the articles. Thank you so much.
Good to hear Ricardo! Thanks for sharing your experience!
Very glad to hear of your daughter’s positive experience with high-dose thiamine. Thank you for sharing. Jeff
I have EDS, celiac disease and SIBO. Have been ill for years. Also diagnosed with CFS/FM, but who knows if it isn’t the other stuff? But I KNOW FOR SURE that I have or get a thiamine deficiency when my SIBO acts up, which it does regularly. I start trembling, jumping out of my skin at the sound of a pin drop — it is obvious, inescapable, that something is wrong. One morning, when I sat down to breakfast with my son (this is more than 10 years ago), I had a glass of OJ in front of me. And I started what I call “the trembles.” I said, “Oh, no, I’ve got the trembles.” And I drank that glass of OJ — which for many years, something like 20 years, I had never been able to drink slowly; with OJ in my hand, all I could ever do was gulp it down as quickly as possible — and within seconds, the trembles stopped. I went to get the OJ carton and see what was in OJ, becAuse it was obvious to me that it was the OJ that stopped those trembles. Hmm, potassium and thiamine. Not a huge amount of thiamine, but it seemed like that was enough, as I had taken huge doses of potassium on many occasions that did not stop “the trembles.”
What is slightly funny is that I had forgotten that my mother, who at the end of her life had slipped in Wernicke’s encephalopathy — something one gets with celiac disease, which they never diagnosed in her, although it turns out that’s what it was — they prescribed injections of thiamine, or B1, to keep her out of that coma. Hmm. Then it took me 20 years to realize that thiamine and B1 were the same vitamin? That just points to my stupidity, but yes, thiamine stops the trembles. But taking thiamine does not. Drinking OJ does, or eating something with lots of thiamine will. But taking the vitamin DOES NOT WORK. How do I know it’s not the potassium? Because I have a huge problem with potassium, and I take it all day long every day, because if I don’t I stop urinating and swell up, a lot in my lower legs, ankles and feet, and become seriously short of breath — which causes intense anxiety. Believe me, I have spent a lot of effort and time on these things, and hope the new GI doc will help me out here. If there is some other vitamin or nutrient in OJ that is never listed, would somebody please inform me? But I believe it is just I can’t absorb vitamins in the form that we take them; only in food. And since I don’t eat that much — I’m already way overweight; why would I eat a lot when I don’t have the appetite? — it seems I cannot get enough from my diet, especially when in the up-cycle of SIBO. After an antibiotic, it all goes away for a while. But then it comes back. Always.
I know I suffer from a thiamine deficiency when my SIBO is cycling up, because I can only stop trembling when I take LOTS and LOTS of thiamine. I take it all day long every day. My understanding was/is that when my SIBO acts up, I cannot absorb the nutrients I need. I get symptoms of many deficiencies. In addition to trembling and jumping out of my skin at the smallest sound, my hair falls out, I get sores at the corners of my mouth and on my tongue, my nails break off at the quick, I can’t sleep, I am depressed, having lost interest in everything that usually engages me…. And I’m sure I’m forgetting some. No appetite. Can’t eat much. Got fat anyway. I have Ehlers-Danlos, celiac disease and SIBO. Thiamine is critical for me, but so is potassium (I can’t pee unless I take tons of potassium), magnesium (I bathe in lots of epsom salts), B-12 (I can’t get enough air, feel like I can’t get a deep enough breath), and I assume everything else. Since taking a liposomal B complex many times a day for months, my nails are growing again and not breaking off at the quick. But I am still short of breath and tremble periodically.
I forgot to mention that I was also diagnosed with CFS/FM, or if you prefer, CFIDS/FM, back around 2000. At that time I also had sleep apnea and periodic limb movement disorder. Both of those syndromes have cleared up. No more sleep apnea; no more periodic limb movement disorder. But I do still get restless leg when I have not been vigilant enough in taking all those vitamins every day, multiple times a day.
Great article!
Been taking high dose bentfotiamine for several days- 1500 mg- it is doing nothing to help with exhaustion or my breathing (i have mild copd and experience ‘air starvation’ frequently- )
I suffer classic PEM symptoms and Chronic fatigue- with weakness settign in 2 days after trying to do any kind of physical work– even brushing teeth on bad days is exhausting and results in a burning muscle pain much like when someone overworks to the point of muscle pain- Unrefreshing sleep- sleep 16 hours a day and can’t ‘catch up on sleep’- I’ll keep at the high dose B-1 for a few months- but really tired of spending $$ on things that promise to reduce fatigue only to fail- and only result in making me poorer- am also taking B-Complex- and sublingual B-12- about ready to give up on all vitamins as nothing seems to make an ounce of difference- Am a bit of complicated case- have Crohn’s, iliostomy- asthma, copd- so lots going on but the symptoms of Chronic Fatigue and PEM fit to a “T”- especially the weakness happening a day or two later as my body ‘crashes’ and has to recover for a few days to get back to normal crappy feeling self-
Thank you so much for this informative article. Your emails bring me hope for the future.
about 20 years ago, I stumbled upon thiamine HCl injections. I was seeing a specialist in nutritional medicine, who was willing to try things like high dose folic acid and other B vitamins, in treating my CFS. I was also receiving hydroxocobalamine injections at the time. I found that 100mg thiamine hydrocholoride, taken intramuscularly, gave me a real boost in energy and mood. And it really helped alleviate brain fog and other cognitive symptoms like poor memory. As it was really inexpensive, I tried injecting myself daily with 100 mg and was surprised with how envigorating it was. At the time, I didn’t need a prescription for it but that changed and eventually, I found it difficult to find a physician who would prescribe it.
Maybe it’s having a vasodilating effect, and increasing blood flow to the brain. We already know anecdotally that some people feel better on vasodilators, some feel worse, and some feel no effect.
Hi Jeffrey,
Has there been any progress with the various hypotheses since this blog post went up?
I recently trialled high dose thiamine, b9 and vitamin A together, as part of an ongoing targeted gut microbiome hack. On day six of this combo, I felt amazing – emotionally, physically and cognitively. Lots of energy and it was easy to regulate my behaviour (since getting CFS 7 years ago, periods of high energy are usually times where I also lose focus and end up overdoing it).
Since reading about thiamine today, I’m sure it was the 1750mg daily dose that was responsible for the increase in energy. My notes show that I was also having issues with sleeping, probably due to taking 1000mg in the morning and 750mg at night. So from today I’m putting the microbiome hack on hold, and will just take the daily high dose thiamine.
If you have any resources, insights or updated hypothesis to share, that would be excellent.
Cheers
Andrew
Has there been any update on B1 causing kidney stones… I am predisposed to calcium oxalate stones, but was unsure of what impact thiamine can have on other stones. I really don’t want to risk that… despite my predisposition, I’ve still not had any stones, but I do a lot of things to prevent them.
The concern about high-dose thiamine possibly contributing to kidney stones is rooted in its property as a carbonic anhydrase inhibitor. I looked into this a fair amount a few years ago and concluded that the risk could be minimized / avoided by drinking extra water. I have not looked into this again. And I am not an MD. So this is just my unprofessional opinion based on the research I did at the time.
I’m wondering if anyone reported a side effect of slowing of intestinal motility? Tried it by 3rd day I was up to 1500 mg which is higher than recommended and I experienced a shut down of my migrating motor movement. This shouldn’t happen cause B1 is actually supposedly cholinergic. I tried this twice with same issue. I have a dx of MCAS as well as ME CFS and EDS. Personally noticed no improvement in sx either time I tried it . May not give up yet. May wash out then try stopping after building to 1200mg then see if I can tolerate at that level for a week or two.
U will not believe what b1 has done for me it’s been ,5 days high dose omg!!! Bad nerves in feet 90 percent better I’m a cancer patient of throat cancer b1 has healed my body of after radation effects my toe nails have growed back cracked heels gone swelling ankles gone blurry vision gone edema in belly gone edema in my neck from RADATION gone planatar fiitis better my inflammation has went down by 80 percent my breathing is deeper lots fluid in both legs gone my skin has the oil back on it this is a miracle from GOD MY NAME IS TAMMIE BOSWELL