

Why? For one – they do seem to be linked together and those linkages could tell us much about each. Despite David Kaufman’s initial focus on ME/CFS, his and Dr. Ruhoy’s Unraveled podcast series is not on ME/CFS – it’s on “Understanding Complex Illnesses“. Bela Chheda and David Kaufman are most known for their work on ME/CFS, but they created “the Center for Complex Diseases“.
Similarly, when #MEAction chose to put their resources into a survey, they created a “Chronic Ilness Survey” featuring ME/CFS but also POTS, long COVID, hEDS, and MCAS, and planned to include more illnesses in the future.
There’s power in numbers. These diseases tend to affect a lot of people, but most get lousy funding. The more groups we have working together, therefore, the better chance we have to get more funding for all of them. The Long COVID Alliance the Solve ME/CFS Initiative cofounded contained illness groups from the ME/CFS, long COVID, dysautonomia, fibromyalgia, autoimmune, migraine, pain and neurological disorders communities. They all recognized they can do more together than apart.
Lastly, the Solve ME/CFS Initiative and others are working to create a post-infectious disease institute at the NIH. The more we can demonstrate the need to study these diseases together, the better chance we’ll have to produce that. We thought that long COVID would lift all boats – and it has – but not at the National Institutes of Health. Lyme disease funding remains strong, but ME/CFS and fibromyalgia funding has either fallen (ME/CFS) or remained flat (FM).
It was very good, therefore, to see two German researchers, Klaus Wirth and Mathias Lohn, back with a hypothesis paper that shows how these diseases may be linked together.

Like a dog with a bone, Klaus Wirth has been digging away at ME/CFS and related diseases. The more he’s looked, the more he’s found.
A Dog With a Bone
Klaus Wirth, it seems, just cannot be stopped (not that anyone is trying). A pulmonologist by training, he’s dug into ME/CFS like a dog with a bone. Over the past 3 years, Wirth has published 5 hypothesis papers that extend what he and Carmen Scheibenbogen proposed is happening in ME/CFS in 2019.
The deeper and wider he looks, the more he seems to find. A hypothesis that originally focused mostly on ME/CFS has, over time, been potentially extended to fibromyalgia (FM), idiopathic intracranial hypertension (IIH), mast cell activation syndrome (MCS), endometriosis, postural orthostatic tachycardia syndrome (POTS) and small fiber neuropathy (SFN).
The Oxygen Crunch
Wirth’s latest paper, “Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Comorbidities: Linked by Vascular Pathomechanisms and Vasoactive Mediators?“, co-authored by Mathew Lohn, asserts that problems with the blood vessels could underlie all these diseases.
The basic idea goes something like this: damaged mitochondria stop the muscles (or brain or other tissues) from rising to the challenge. Since energy is, after all, the currency of the realm – our bodies, above all, need it – the muscles or other tissues put out all the stops to get more energy; i.e. to bring more oxygen in. The way to do that is to increase blood flow to them.
There’s a problem, though. At the same time the mitochondria have pooped out, Wirth and Scheibenbogen posited that an ongoing sympathetic nervous system (“fight or flight”) hyperactivation has clamped down hard on those blood vessels – preventing blood to freely in them. Recent studies suggest that they’re correct.
A process called stagnant hypoxia that may exist in ME/CFS and POTS provides another way by which oxygen delivery may be impaired in these diseases. Stagnant hypoxia occurs when the oxygen content of the blood is normal, but the blood is moving too slowly to deliver sufficient amounts of blood to the tissues.
The idea that oxygen-starved tissues are causing pain and fatigue in ME/CFS and fibromyalgia goes back at least ten years.
David Systrom’s findings of impaired oxygen extraction at the level of the muscles would seem to cement this issue.
The Vasodilator Dump
While it’s apparently impossible to test for these substances (they disappear too quickly), Wirth, Scheibenbogen, and Lohn appear to have a solid basis for their hypothesis proposing that the body must be dumping massive amounts of vasodilators to open up those blood vessels and restore oxygen flows to the mitochondria in ME/CFS.
That might be all to the good in acute situations, but in chronic situations like ME/CFS, the vasodilators spill over into the bloodstream, causing pain and all sorts of symptoms.
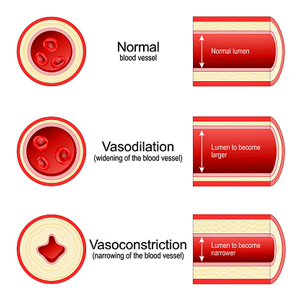
The authors believe energy-starved tissues release massive amounts of vasodilators in an attempt to open constricted blood vessels in ME/CFS and related diseases.
That process – where tissues deprived of oxygen fight back by releasing vasodilators is known to happen in the heart. In ischemic (low oxygen) heart disease, our hearts pump out a variety of mediators (prostaglandins, prostacyclin, ATP, adenosine, and particularly bradykinin) to get the blood flowing again. The downside is the tissue acidosis, pain (angina), blood vessel leakage) they cause as well.
The Gynecological Connection
The gynecological connection to ME/CFS has received surprisingly little attention in a disease that’s dominated by women. Several studies, though, have found higher-than-normal rates of gynecological diseases in ME/CFS. Indeed, they suggest that as many as a third of all women with ME/CFS may suffer from endometriosis and resulting comorbidities.
Nobody, though, has potentially connected the blood flow issues in ME/CFS to the gynecological issues until now. The authors propose that a muscle/blood flow problem is either contributing to dysmenorrhea/endometriosis in ME/CFS or, interestingly, is perhaps causing ME/CFS in those diseases.
Dysmenorrhea
In dysmenorrhea (painful menstrual periods), we see a familiar situation; hypercontractile muscles lock down blood flows, causing an oxygen deficit (ischemia). That, the authors believe, triggers a massive production of vasodilators like bradykinin and prostaglandins to open the blood vessels back up and restore the blood flow. (The authors suggest the process may be akin to having an “intravenous infusion” of a vasodilatory “cocktail”.)
Once again, the authors propose that these powerful, pain, muscle spasm, blood vessel leak-producing compounds are spilling into the bloodstream and causing pain and other symptoms.
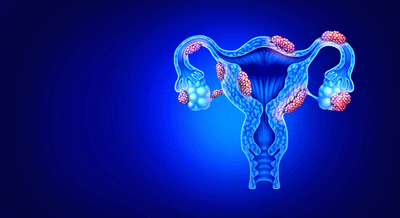
In endometriosis, tissue grows outside the uterus.
Endometriosis
Endometriosis is a different case. Endometriosis (EM) occurs when endometrial tissue grows outside the uterus, triggering a “chronic inflammatory, estrogen-dependent gynecological disease”. Endometriotic tissue can be found in quite a few areas outside the uterus (peritoneal cavity, ovaries, bladder, or ureters); the effects can be widespread and quite complex.
THE GIST
- People with chronic fatigue syndrome (ME/CFS) are not alone. Nor are people with fibromyalgia (FM) or dysautonomia or migraine. We all exist in a swirl of allied illnesses. Doctors recognize that, but researchers who typically get trapped in silos generally have not.
- Recognizing the connections between these diseases and studying them will help us understand all of them, and importantly, should increase funding for these typically greatly underfunded diseases.
- Given that, it was great to see two German researchers, Klaus With and Matthias Lohn, band together to produce a hypothesis paper potentially linking ME/CFS, endometriosis and MCAS together.
- They propose that massive outflows of vasodilating substances that are spilling over into the bloodstream are producing the symptoms in these diseases.
- In ME/CFS, mitochondrial problems are limiting energy production, causing the body to find ways of delivering their “food” – oxygen to them. This requires opening the blood vessels to allow more blood through. The problem, though, is that the blood vessels in ME/CFS are already tightened down – requiring massive levels of vasodilating compounds to open them.
- Too high levels of these compounds (bradykinin, PGE2, and others), though, can produce pain, fatigue, other flu-like symptoms, and sleep disturbances; i.e., most of the symptoms in ME/CFS.
- The problem is exacerbated by the inability of adrenoreceptors (β2AdR) to open the blood vessels and support mitochondrial health.
- Wirth and Lohn propose that a similar process occurs in the gynecological disorders associated with ME/CFS (painful menstrual periods, endometriosis); they believe that contracted muscles lock down blood flows, causing an oxygen deficit (ischemia) which results in the production of massive amounts of vasodilators that produce the painful menstrual symptoms.
- In endometriosis, they propose that a vasodilator called prostaglandin E2 (PGE2) causes the blood vessels to dilate; it’s also causing the uterine tissues to contract – thus imperiling blood flows and producing a kind of contraction/dilation back and forth. Instead of this process causing endometriosis, they believe it’s causing women with endometriosis to become diagnosed with ME/CFS.
- Finally, in mast cell activation syndrome (MCAS), they believe an overabundance of vasodilators like histamine is producing similar symptoms to those found in ME/CFS, endometriosis, and dysmenorrhea.
- Next up – a further look at the connections underlying these conditions as we tackle a paper and NINDS webinar produced by Beth Pollack.
PGE2 causes the uterine tissues to contract and vasodilates (opens up) the blood vessels. That muscle contraction stops enough blood from getting through – which leads the tissues to produce more PGE2 production to open the blood vessels – but which also causes the tissues to contract more.
PGE2, interestingly, is not just a pain producer. It’s also been linked to the familiar flu-like symptoms, fatigue, and sleep disturbance found in ME/CFS. The authors proposed that it’s the PGE2 production in endometriosis that’s causing so many women with that condition to become diagnosed with ME/CFS. Overly high levels of PGE2 production, in other words, may be jumpstarting these conditions.
Mast Cell Activation Syndrome (MCAS)
The authors propose that a similar but different process occurs in MCAS. In the MCAS scenario, hyper-sensitive mast cells blast damaging mediators (histamine, heparin, prostaglandins, leukotrienes, and different proteases (tryptase, chymase), and more than 30 cytokines and chemokines) into the blood.
Here, histamine probably plays the same role in MCAS that prostaglandin E2 does in ME/CFS and endometriosis. It’s spilling into the bloodstream, causing pain, fatigue, flu-like symptoms, sleep issues, etc.
In each of these cases, high levels of vasodilators cause blood vessels to leak, produce spasms, and affect the sensory nerves – causing pain, fatigue, flu-like symptoms, etc.
The B2ADR Connection
Problems with the beta-2 adrenoceptor (β2AdR) figured strongly in Wirth’s and Scheibenbogen’s original hypothesis. In some ways, the β2AdR is the perfect receptor for ME/CFS. (Receptors dot the surface of cells; when they get turned on, they tell the cell how to act.)
Not only does the β2AdR play an important role in regulating blood flows to the skeletal muscles, the heart, and the brain, but in a nice twofer, it also affects cellular energy production.
This is because the β2AdR receptor stimulates the Na+/K+-ATPase enzyme that controls sodium levels in the cells. A failure of the β2AdR to trigger Na+/K+-ATPase can cause sodium buildup in the cells, which causes the sodium-calcium-exchanger (NCX) to switch sides, so to speak, and start importing rather than exporting calcium.
Not only does this calcium buildup damage the mitochondria but it can also injure the endothelial cells lining the blood vessels. Therefore it offers a potential double whammy: messed up energy production and malfunctioning blood vessels – both of which can be found in ME/CFS.
With that, we’re back to square one – we have energy-depleted muscles and a broken vasculature. The next step is now a familiar one with oxygen-depleted tissues. It involves flooding the muscles with vasodilators which then spill over into the blood – and potentially symptoms of pain, fatigue, flu-like symptoms, problems with sleep, etc.
Because β2AdR just happens to play an important role in uterus relaxation, dysfunctional β2AdRs could contribute to increased levels of uterine contractions which inhibit blood flows, thus starting the vasodilation process off.
Lastly, some evidence suggests that the β2AdR helps stabilize mast cells – suggesting that balky β2AdRs in ME/CFS could trigger MCAS.
Drug Effort
Wirth has found a drug that he believes could help clear up the muscle/blood vessel mess and has formed a company called Mitodicure to promote it. Mitodicure is currently looking for funding.

Klaus Wirth reported that recent findings highlight the muscle/mitochondrial connection in ME/CFS. A blog is coming up on that.
Conclusion
Wirth and Lohn have added another addendum to their 2019 Wirth/Scheibenbogen hypothesis. Time will tell, of course, whether they’re on the right track or not. Simply by making us aware of the possible connections between ME/CFS and the comorbid conditions it is allied with, though, they’ve done us a service.
Wirth also reported that recent findings highlight the muscle/mitochondrial connection in ME/CFS. A blog is coming up on those, plus Health Rising is going to continue to explore the connections between all these “complex illnesses”. Coming up shortly, Beth Pollack explores the reproductive and connective connections between ME/CFS, POTS, connective tissue disorders like Ehlers-Danlos Syndrome (EDS), and endometriosis in a review paper, and leads a NINDS webinar exploring those connections.


 Health Rising’s Quickie Summer Donation Drive is On!
Health Rising’s Quickie Summer Donation Drive is On!



All very nice, but are they looking at drugs that could improve these issues?
Good question! The answer is yes! I should have put this in the blog (and will). Wirth’s Mitodicure company believes they have a drug that can help and are trying to raise funding for it.
Hi Cort, I just looked for a website or way to donate but can’t see the right thing. Do you know at all? Thanks for your brilliant blogs!
It’s at the very bottom of the article, above the comments, in rather small print, at least on my phone.
Thanks Sue, sorry I meant for Mitodicure!
While I kinda love the papers written by Wirth and some of them struck a cord with me, I am sad to report that after contacting him, he is in no way interested in helping us a as a community it seems.
A friend and I contacted him, she had a special ultrasound that probably confirms some of his hypothesis. The answer was short, rude and in no way helpful. He didn’t seem interested in the info we provided or interested to act on this info.
I am sad to say, that I beliebe this again is just all about the money for the few, instead of help for the many affected.
Sorry to hear your friend had this experience! I would be careful, though, about drawing general conclusions about his motivations from one interaction. My experience with Klaus has quite different – he’s always been very accessible and helpful. He did a great deal of work on ME/CFS prior to forming Mitodicure.
I hope Mitodicure can be absorbed into a large, established execution group with funding. Despite the months passing since Mitodicure’s listing, their company still looks quite small (2 people?) far far from operationalizing trials and treatment.
Yes – as we’ve seen with Cortene finding funding for this disease is still a challenging proposition.
Hello Cort, I have ME/CFS and know people interested in giving to solid ME/CFS research. How do I get in touch with Prof Klaus to learn more? Im happy to pass contact info along to you. Im fortunate enough to know people with very deep pockets willing to donate.
I’m so glad we have Wirth et al with our communities – he seems seriously committed to ME and linked diseases and his hypotheses make so much sense to me.
I’d be interested to hear the experiences of people with ME who take midodrine for POTS? It seems counterintuitive to this hypothesis since it’s a vasoconstrictor which aims to push the pooling blood in the legs back up and stop blood pressure being very low. I’ve just started it and it’s helped me be upright for a little longer (practically bedbound so it’s tiny steps) but I’ve also experienced far more aching limbs and a lot of shortness of breath amongst other negative side effects. I’m wondering if that ties in with the ischemia part…?
Hi Chips,
I have been prescribed midodrine for my POTS and I also have severe ME, Fibromyalgia and MCAS. I have seen positive results with the midodrine. I’ve been taking 10mg in the AM and 10mg at bedtime for the last year. I am able to be upright more without having really bad symptoms. My cardiologist said that they aren’t exactly sure why this med can be helpful for people with POTS. I have already had chronic pain prior to starting this medication, so I don’t believe that it has increased or decreased my pain. I just make sure to also drink 5-6 liters of electrolyte water all day. I also add 1/4 teaspoon of pink salt to each liter of water and I eat higher sodium foods and add extra salt to whatever I’m eating. I do notice a increase in pots symptoms if I don’t keep up with the electrolyte water like I should. I hope this helps you at least a little!
Peace and love,
Mel
Hi Mel, thanks so much for posting your experience, it’s really helpful to read and I’m so glad you’ve had such positive results with it. I will have to try electrolytes too!
Thanks for this article, Cort. Research from the endometriosis side of the equation noted the endo/CFS connection in the ‘90s, with findings indicating that women with endo were **100 times** more likely to get ME/CFS than women without. The endometriosis association included this info their research summary. I’ll see if I can dig up the citation or lead PI’s name.
Wow! I Would love to see that. I didn’t know that the ME/CFS association with endometriosis had been approached from the other direction. That’s what Wirth and Lohn predicted or reported, though.
In fact, I’ve hardly heard about this association before – which seems so weird given the female predominance in ME/CFS that starts showing up, if I have it right, during the hormonal changes that occur during adolescence. It’s good to see it getting some light. I’m looking forward to seeing what Beth Pollack says about it.
Not surprised by this finding at all. I had a five-hour hysterectomy (TVH-BSO) at 28 for severe endo that Danazol didn’t help. About 7 years later, I was diagnosed with fibromyalgia and ME/CFS a decade after that.
What really drove this home for me was an unusual experience I had at the CFS/FMS Conference in 2002 at a hotel near LAX airport. When we broke for a catered lunch buffet, I sat at an empty table and 9 other women, mostly middle-aged, joined me. While we ate, we discussed our various war stories dealing with our conditions. Turned out that 8 of us had had a hysterectomy by age 30 for severe endo! The endo came first, then drugs like Danazol that didn’t help, then the hysterectomy. We all had our jaws on the table.
In any other setting, this coincidence would have been beyond belief. But at a CFS/FMS Conference? Yeah. I get it now.
Lynne, I also have endometriosis and found those stats related to endo. It amazes me that no one has really pursued this more, or that we don’t have any connection with the endo community.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10806170/
I hope you find this article interesting. It encompasses intracranial hypertension and Ehlers-Danlos/hypermobility and blood vessel issues. It’s written by Dr Kyle Fargen, a neurosurgeon who’s putting in brain stents for Ehlers-Danlos patients, most having intracranial hypertension.
Thanks!
That is very interesting indeed. At last someone is talking about MACAS.
This was definitely a key part of my ME/CFS when I had it but went away once I recovered. I was ‘allergic’ to just about everything and covered with hives, coughing eyes streaming etc. . I could ‘write’ on my skin with my finger.All sorts of ‘allergic’ reactions. I had an episode of anaphylaxis, which I survived. Called hihgly reactive and etc etc.
At an INVESTINME conference in London an American doctor said to me you have MACAS. I had never heard of it before but once I looked it fitted, or at least it did during that period of my illness. I remember being so ‘allergic’ my eyes were streaming and swollen that I could not see.
I cleaned out just about everything in my life that had anything to do with ‘allergy’ ( ie extreme reactions) but also cleaned out my troubled personal life and a couple of years or maybe 3-4 years later I realised I wasn’t ‘allergic’ any more and the swelling and chocking and so forth had cleared up.I continued to occasional hypoglycaemia episodes but learnt to manage those, well more or less.
Thank you for sharing this information on Mcas! Super helpful! Ive been battling MCAS for a few years now and talk about it on my blog http://www.damngoodthyme.com hope you check it out 😊
maybe you could approach social entrepreneur Erik Stangvik for fund raising??
Maybe Lady Gaga has contacts that could help fund Wirth’s research. She apparently has FM and migraines. She is going to have a show on Netflix about her ordeal.
I have (or have had) all of these conditions. My ME/CFS began when I was given hormonal treatments for endometriosis (which -a- I was never given proper info for informed consent and -b- never helped the endo). I’ve had MCAS my whole life, although not always activated. (In early childhood, I now realize, and then again after ME/CFS took hold.) I’m 65 now. After 35 years of trying to find “cures” I’ve improved somewhat, but by now I’ve given up “the search.” Just trying to live the best I can with remaining years.
What do you think has helped you the most to improve?
For MCAS, 2 things: (a) Most important – moving to a place with clean air. We succeeded in finding a house without mold (not easy), I learned to avoid chemical cleaners etc. And I can (finally) breathe clean air. (b) I improved a lot, and then I found out about ketotifen, a mast cell stabilizer, which I take regularly now. I feel that it is slowly rolling back the sensitivities which had been gradually becoming more sensitive with every exposure. (I do not believe it would work if I did not already have clean air, clean environment.
Thank you for this post, Cort.
However, I think that the hypothesising of Klaus Wirth rather serves to bring confusion to the understanding of the pathomechanism of ME/CFS than more clarity.
First, I have no idea why we should theorize a medical connection between illnesses that are underresearched. Who gets the money to research what depends on political processes. Here the connection between fibromyalgia, ME/CFS, migraine, and endometriosis is evident. Fibromyalgia is a gynecological illness, the others present themselves about 3 times more in women than in men. Therefore these illnesses have a long history of being not taken seriously but mistaken by the misogyinist Western academic medical field for women’s mental health issues or simply as a prove of women’s insanity and inferiority to men.
If you don’t know anything yet about the prevasive aversion against women in the medical field you might want to watch “Unrest” by Jennifer Brea about ME/CFS as an introduction or read this article about the work of one of the leading migraine researcher Dr. Peter Goadsby:
https://www.theguardian.com/society/2021/oct/09/can-migraines-be-untangled-by-new-medical-thinking
If you want to dig in into the theme more I recommend “Unwell Women” by the British historian Elinor Cleghorn that was published just two years ago. She works out how the medical field is an important agent in forming and maintaining patriarchal rule and how this leads to women’s health issues chronically underfunded. It is a tough read but at the same time a beautiful an important book.
Here is an article about Cleghorn’s book: https://www.theguardian.com/books/2021/jun/08/unwell-women-elinor-cleghorn-book
Second, I don’t see that this wild theorizing of Klaus Wirth on the pathomechanism of ME/CFS does lead anywhere. As a person with a degree in history, philosophy and art history Klaus Wirth’s work strucks me as merely speculative.
In philosophy thinkers from that realm are called metaphysicists because they are not interested in formulating well formulated simple questions that they then will test empirically but they are interested rather in story telling, explaining things that cannot be tested. It’s ok and can be valid to do that. Sometimes we need to have answers. Many people for example want to have an explanation what happens to them after death, a classical metaphysical question, but it is not the scientific method.
However, there is very good research in ME/CFS that is able to formulate these clear and simple questions that then can be tested empirically. And from this research we already know so much.
It was already clear in 2019 that the damage to the mitochondria came from something upstream as is summarized in this paper by ME research UK:
https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjNgKqMkp6EAxVnzgIHHajgAVEQFnoECCYQAQ&url=https%3A%2F%2Fwww.meassociation.org.uk%2Fwp-content%2Fuploads%2FMEA-Summary-Review-The-Role-of-Mitochondria-in-MECFS-12.07.19.pdf&usg=AOvVaw0mUVXNIjKnMY_Y8q7oohwf&opi=89978449
In 2020 Bhupesh Prusty in his Nature article was able to show that early enzymes in HHV6b reproduction were just that missing piece in the puzzle.
https://pubmed.ncbi.nlm.nih.gov/32327453/
Many more researchers have contributed to bringing the question of herpes reactivation in ME/CFS to the test and every new study brings more evidence that this is the case and more clarity into this solvable puzzle. (In contrast to the questions that researchers like Klaus Wirth formulate that are neither simple, clear, nor testable).
What you might have missed, Cort, is that in January a Long Covid study from immunologists from Zurich was published in Science that hints to the fact that it is a special part of the immune system, the complement system, that remains active in Long Covid (and as they theorize possibly ME/CFS) and goes back to normal in LC patients whose problems stopped after a couple of months. (Here in Europe it was widely discussed and interpreted as a break through by many media outlets).
https://www.science.org/doi/10.1126/science.adg7942
Just as other immunologists do the researchers theorised an overactive immune system and possibly autoimmunity. But they also mentioned that they had strong signs that this immune reaction was possibly triggered by herpes reactivation.
What I found particularly interesting, and here I come back to Klaus Wirth’s blood vessels question: The researchers from Zurich explain that a continually activated complement system explains the damage to the “red blood cells, platelets and blood vessels”.
https://www.news.uzh.ch/en/articles/media/2024/Long-Covid.html
More, the complement system is that system of the immune system that is responsible for reactions against viral and bacterial infection. Which indirectly supports the idea that there is a (herpes) virus infection going on in Long Covid and ME/CFS.
In sum, the hypothesis of herpes reactivation is not only simple and clear but it can also explain the episodic (and inflammatory) character of ME/CFS. It has over the last years brought forward evidence how the mitochondria is damaged and why the typical PEM with patholgical exhuastion occurs. And with this latest study on the actication of the complement system and the damage to the blood vessels that it causes we have further prove that ME/CFS might have a very simple cause in HHV6b reactication.
Ockham’s razor 🙂
Yes, Gijs. I also thought of this lately when I realised that so many people in the ME/CFS field believe that the more complex and difficult and interconnected we describe the problem the more progress we will have. Nothing could be further from the truth. Ockham’s razor is such an important principle to guide one’s thinking!
I think you wil like this study Lina.
https://pubmed.ncbi.nlm.nih.gov/36300129/
This is just one of many studies about HHV6 , EBV and autoimmunity.
It has been found many times in ME/CFS/POTS.
So Karl Popper 🙂 would say: it comes closer and closer to the truth…
A question for you: does HHV6 or EBV cause a disruption of the immune system with all its consequences or can the immune system not properly control these viruses? These viruses are also known to reactivate under stress.
Thanks for replying. I have to pace and prioritize other tings in the next days but I hope I can come back and think about that question that you raised.
Thanks for the link to this article, Gijs. I didn’t know it yet. It seems that these researchers are from Eastern Europe and Russia mainly. This is great because they are good in herpes research.
One of my professional health supporters with parents from that region made me aware that the Russians seem to have a treatment against HHV-2 and the debilitating herpes genitalis it causes. Since ME/CFS has just the same outer form with its “characteristic persistent remitting and relapsing nature of the ME/CFS syndrome in many individuals”
https://www.brunel.ac.uk/research/projects/reactivation-of-herpesviruses-in-chronic-fatigue-syndrome
this could be a path to the development of drugs.
Regarding your question: I have very little understanding of EBV I have to admit. So I can only talk about the HHV-6b hypothesis in ME/CFS.
To me, what Bhupesh Prusty theorises about these questions sounds the most interesting and most promising.
https://www.youtube.com/watch?v=0dI7uaTni5g
The main reason is that he has not only an interest in his molecules but he is extremely well infomerd also of the clinical presentation of ME/CFS. Especially, he understands that there is (in some patients) only a mild level of illness and that at that stage it is quite normal that people go back to being cured when they pace and rest for a couple of months.
Clinically, the mild level is described best here: https://www.youtube.com/watch?v=EUIdbbwcnAE
And very importantly the doctors from this institute even present a daignostic procedure for the mild form.
I think that this is something extremely important to know because at that level patients do not fit the Canadian Consensus Criteria or other diagnostic apporaches when at the same time the ailments they present with already have that specific mark of ME/CFS.
I had this slow entry into the disease myself and when I got worse and my symptoms had progressed into a smoldering, “mild” form of frontal lobe brain inflammation which looked like this: https://www.tagesanzeiger.ch/andrea-salvisberg-hirnhautentzuendung-und-spitzensport-973838521004
and only then found out that I had ME/CFS I was extremely angry that no one could have told me that I needed to pace and rest. But I am losing track of your question…
Since for many patients the trigger isn’t a viral infection I wouldn’t focus too much on those for the onset of the disease. It seems that different paths lead into a mild or directly into a moderate form of ME/CFS.
Since I believe that smoldering (the full blown meningoencephalitis often ends with the death of the patients) HHV-6b reactivation and brain inflammation ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7912523/ ) is the pathomechanism of ME/CFS and not only plays an important role I would theorize that many different events can bring a change to the immune system and has the effect that it is not capable anymore of controlling smoldering HHV-6b inflammation.
On the rest of the processes I have to read up first. For example it is known that HHV-6b is “lymphotropic”. I think that means that it likes to replicate in cells of the immune system… Just yesterday I posted an article that showed that HHV-6b reactivation is facilitated by a lack of T-cells.
Thanks again for your question! I think I want to focus on bringing forth more arguments that episodic, smoldering HHV-6b reactivation is the best scientifically substantiated explanation that is available right now and that we should have therefore the most money going into researching this question.
When my health allows I hope I can do more about that.
Last but not least: I have written about this before, but maybe you are not aware. The reason why I am 100% convinced of the smoldering HHV-6b reactivation hypothesis in ME/CFS is because when I realised I had possibly some form of herpes encephalitis I suspected that I might have Mollaret meningitis, a not lethal, self-ending form of HHV-1 brain inflammation. Thus I pressed my Ear Nose Throat doctor to prescribe me aciclovir on speck – and it worked and stopped the inflammation process immediately. After tapering off after two weeks I didn’t even have PEM anymore when I severly violated the energy limits that I had had for more than two months during the episode. Plus, I have talked to one doctor who prescribes herself aciclovir herself and goes on working and experiences just the same symptoms and drug effect that I had. (Aciclovir has good activity against HHV-6 in vivo)
I was pretty angry at first that my family doctor denied me aciclovir when I had the next episode about four months later after a period of experiencing more distress than normal. However, given that it is pretty toxic and I was able to learn to rest and pace well and haven’t provoked a relapse within 13 months now and are recovering slowly but gradually I am glad!
Thx for your explanation. Nice that you are slowly recovering.
I wish i had pace myself at the beginning of my disiease.
My doctor told me to exercise and go on so i did.
It maked me so sich i ended up in a wheelchair and was bedbound for years!
Thank you, Gijs, also for your other reply about HVV-6b. I found that very helpful and will come back to the discussion when I can.
I am sorry that you got so much worse with the recommendations of your doctor. It is difficult to even hear these stories. It’s so tragic to be derailed by well-meaning and highly confused MDs. Many of them are a real danger to humanity. How is your recovery going? And what helps you?
Yes that complement activation study is very interesting. I commented on it in one of the articles on this website a couple of weeks ago.
That sounds interesting. But I remember that you don’t buy into the herpes virus theory as you once wrote. What is it then that you did you find particularly interesting about the findings of that Zurich study?
Herpes Virus is only one possible explanation for complement activation. I favour a more generalised hyper-immune state unrelated to herpes virus. I could see that herpes could contribute to this state, but is not the singular causative agent.
That is just my view, though!
I will add – something I find very interesting about the complement activation idea is that the complement system is very strongly linked to the mitochondria…
Check this paper out below! How about this quote:
‘The complosome controls mitochondrial activity, glycolysis, oxidative phosphorylation, cell survival and gene regulation in innate and adaptive immune cells, and in non-immune cells, such as fibroblasts and endothelial and epithelial cells’.
So the complement system seems to play a key role in a whole lot of things out of whack in ME/CFS….
https://www.nature.com/articles/s41581-023-00704-1
I will pace well and then come back to have a look at this. Sounds interesting!
Herpes zoster is also cropping up in many COVID patients and those who got the COVID vaccines. And a nastier variety of shingles which tends to attack the eyes, ears, brain, entire system (Justin Bieber, Dianne Feinstein). My unscientific observation is that most of us with CFS/ME have a host of viral and bacterial infections that we are battling. Mycoplasma among them. The immune system gets so worn out that we become very susceptible to just about anything we’re exposed to. Just as this new, strange and undoubtedly man-made COVID virus provokes this, I am convinced ME/CFS was triggered by another such virus. And that there were very strong viruses that appeared in the year or so before big brother COVID appeared, that were never really identified. Disappointing our govt does not want to fund research that identifies these viruses and possible drugs to kill them.
Yes, Linda, there are all sorts of infections going along with the ME/CFS condition. I think I have more viral infections like colds and covid since I am ill, and I constantly have to watch out to not catch skin fungus and gum inflammation.
However, this is a well known side effect of ME/CFS. Because the immune system is weakened there are more such incidents that are more diffilult to fight off again for the body.
This heightend susceptibility to infections is even a factor within the Canadian Consensus Criteria to diagnose ME/CFS. Did you know about that?
The HHV6b (braininflammation) reactivation theory is something different. It aims at explaning the root cause of ME/CFS itself.
The condition which is now knwon as ME/CFS to my knowledge is known in the medical field since several centuries. The most occurring trigger is EBV. So to me there doesn’t seem to go on something mysterious here.
Conceiving a pharmacological strategy for treating ME/CFS and developing it as a drug as a pharmacologist and medical doctor is something very concrete, and not theory. This is what I am doing as a drug researcher. My work includes the testing of compounds, which seem promising on theoretical grounds, on efficacy in concrete pharmacological experiments.
Conceiving a pharmacological strategy for a disease with no effective treatment requires a certain level of understanding of the disease mechanisms that are operative which means it is essential to have a working hypothesis. This is or was the major difficulty.
The heuristic method you are advocating for had been applied for decades; it is even one of the main causes for the delay in recognitions from which patients still suffer so much. The conception for my drug strategy which I try to realize with my company Mitodicure is based on a deep analysis of the disease published in several papers.
Our working hypothesis is that mitochondrial dysfunction and damage together with malperfusion plays a key role for exercise intolerance, PEM and even systemic symptoms as explained in several publications. Our drug is conceived to treat and prevent ionic disturbances otherwise well known form experimental medicine that cause mitochondrial damage.
The key assumption of this hypothesis, a rise in intracellular sodium in myocytes, has been proven by the demonstration of elevated intracellular sodium in skeletal muscle (23-Na MRI study in ME/CFS patients). This study was performed based on our hypothesis. Several papers published early this year prove skeletal muscle pathology, structural damage to myocytes and mitochondria which is a breakthrough in ME/CFS research.
Thank you for your service to us all.
Thank you so much for helping our community we’re lucky to have you!
Klaus, this is very interesting. You are telling us that you thought that a shift in paradigm was necessary. I doubt it but I will think about this.
I have to pace (and prioritize) well so I can’t go on with the discussion immediately but I hope I can come back soon.
The basic idea goes something like this: damaged mitochondria stop the muscles (or ”brain or other tissues) from rising to the challenge. Since energy is, after all, the currency of the realm – our bodies, above all, need it – the muscles or other tissues put out all the stops to get more energy; i.e. to bring more oxygen in. The way to do that is to increase blood flow to them.
There’s a problem, though. At the same time the mitochondria have pooped out, Wirth and Scheibenbogen posited that an ongoing sympathetic nervous system (“fight or flight”) hyperactivation has clamped down hard on those blood vessels – preventing blood to freely in them. Recent studies suggest that they’re correct.’
I think this is a plausible explanation. If I understand correctly, is the overreaction of the sympathetic nervous system a consequence of getting more blood and oxygen to the muscles and all organs? Then we are back to the autonomic nervous system and the overdrive theory. It remains a two-way street.
This remains one of the most fascinating theories.
Er komt steeds meer bij en het wordt naar mijn gevoel steeds onduidelijker!
Het wordt breder en breder , de ene publicatie na de andere over allerlei mogelijke oorzaken en ondertussen verdwijnt de hoop op gerichte medicatie! Ik weet het , er wordt keihard gewerkt, maar hier in Nederland krijgen sommige van ons het idee dat we ondergesneeuwd raken door ziektes die apart onderzocht zouden moeten worden. We/ík voelen ons iig door Long/covid er nog maar een beetje bijhangen.
Sorry dit is waarschijnlijk niet de realiteit maar toch
En Cort super bedankt voor al je werk !
This is so interesting. I developed MCAS symptoms in my mid 20s, migraines in my late 20s, endometriosis requiring surgery in my early 40s, and ME/CFS or Long Covid in my late 60s. And I’m learning now how they are all connected. Absolutely fascinating.
I always thought my hormones ruined my life…..Premature ovarian failure and since than my life went downhill with tiredness, atypical migraines, I was also diagnosed with possible endometriosis but because I went into early menopause they did not further research. I have a long list with symptoms psychological, neurological and physical and replacing hormones did not solve all my issues. I cannot file for disability because I have no diagnose which is taking seriously but I cannot functioning anymore. I live back home and my whole life is ruined and I don’t understand why they never talk about the role sex hormones play.
I don’t have endometriosis. But I did have an injury to my uterus in 1986. My uterus came out with the birth of my son.
Also, I had premature ovarian failure at the age of 38 and my periods stopped.
As a few others have commented here, I also was diagnosed with Premature Ovarian Failure years before my ME developed. I was diagnosed with POF at 16 but felt relatively okay until being put on a high dose of HRT at 26, and my health totally collapsed. A viral trigger then pushed me into full blown moderate/severe ME (though I wonder if I had ‘mild’ ME previous to this from the HRT. I was constantly going back and forth to different Drs begging for help and being brushed off). I know that there is a study showing early menopause is also a risk factor for ME.
This hypothesis’s description of the body flooding with signals to dilate the blood vessels reminded me of a paper on POTS I skimmed a while back. It was about an attempt at modeling the low frequency oscillations seen in HR and BP in POTS. These oscillations have been hypothesized to cause brain fog in that syndrome.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9399868/
I think this is it.
Nice – thanks!