

Geoff’s Narration
The GIST
The Blog
(The GIST is on the bottom right of the blog today)
The “Oxidative Stress is a shared characteristic of ME/CFS and Long COVID” preprint is an example of how a small but well-done study can still reach very deeply into the biology of ME/CFS. Health Rising covered Mark Davis’s presentation on the effort in the Dec 2023 NIH ME/CFS Conference, but the preprint that was just published includes long COVID and has new findings.

Vishnu Shankar, an enterprising graduate student, turned a struggling NIH grant on T-cells into a win.
Some people may remember that Health Rising reported that becoming obsessed with the energy problems found in ME/CFS, Vishnu Shankar, a PhD. Stanford student, engineered a study that assessed energy production in ME/CFS patients’ immune cells.
Shankar focused on immune cells because their energy needs are so high. It turns out that protecting the body from pathogens is quite an energy-intensive project. Even when it’s not fighting off an infection, the immune system uses about 15-20% of our energy. (When it is fighting off an infection, the body will devote about 25% of its total energy expenditure to the immune system.)
The Study
This study did something fairly simple: it assessed both mitochondrial activity and oxidative stress in immune cells (PBMCs) in 16 healthy controls, 15 ME/CFS and 15 LC donors. While doing so, it opened up a world of possibilities.
Mitochondrial Breakdown
While this study did not find reductions in mitochondrial mass or mitochondrial membranes in ME/CFS or long COVID, it did find reduced production of a considerably more important factor – ATP production. The immune cells in the ME/CFS/Long-COVID patients weren’t pumping out as much energy as the healthy controls cells – suggesting they were damaged and possibly exhausted.
Because damaged mitochondria aren’t just bad at producing energy but can become free radical-producing machines, they took a look at oxidative stress (free radical) levels. (Just as a damaged automobile engine produces more exhaust than a well-functioning one, damaged mitochondria spew out more toxins; i.e. free radicals.)
Elevated Oxidative Stress Levels
Immune cells have to switch their after-burners on to get the energy to go after pathogens. Producing that energy comes at a cost, though, in the form of increased levels of reactive oxygen species oxidative stress (free radicals). (Note the key word – reactive oxygen species – these are unbalanced oxygen molecules which try to achieve balance by ripping electrons from other molecules; hence the word “reactive”.)
These reactive oxygen species, or free radicals, are a normal byproduct of aerobic energy production, and our systems have evolved to handle them. But not so well, apparently, in ME/CFS and long COVID. Various studies that have found low levels of antioxidants and increased levels of oxidative stress suggest that antioxidant systems in ME/CFS are not up to the task.
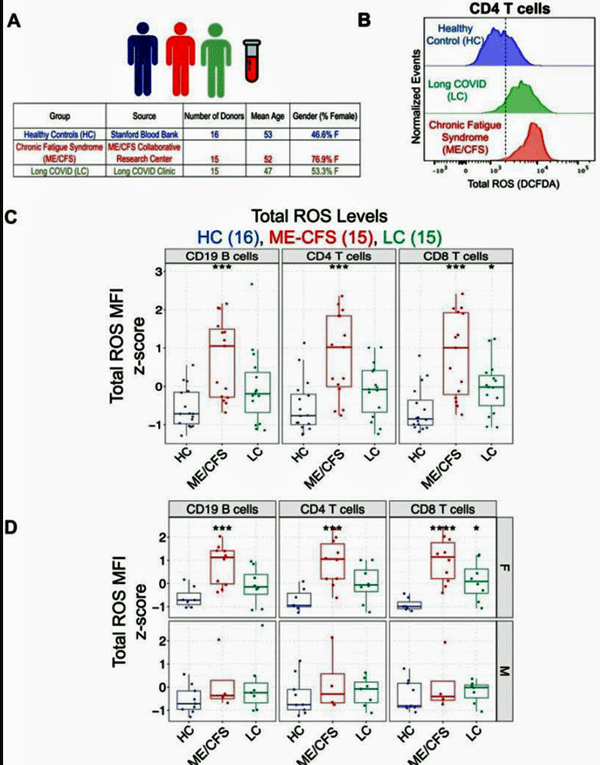
From the study. Note the dramatically increased levels of reactive oxygen species in B and T-cells in ME/CFS patients in particular (ME/CFS – red, LC – green, healthy controls – blue).
Indeed, in this study, dramatically higher levels of reactive oxygen species were found in both the ME/CFS and long-COVID patients’ immune cells compared to the healthy controls. (ME/CFS patients had the highest levels).
A closer look revealed, however, that the reactive oxygen species were almost wholly centered in the immune cells from female ME/CFS patients. Their levels were 2-4x’s higher than the healthy controls. The immune cells from the male ME/CFS and male long-COVID patients did not have significantly increased reactive oxygen species.
Both male ME/CFS and long-COVID patients did, however, exhibit elevated glutathione levels – indicating that their immune cells were also dealing with increased oxidative stress levels – which have triggered the production of the master antioxidant in our cells – glutathione.
Altogether, multiple pathways that deal with oxidative stress (glutathione, superoxide dismutase, lipid oxidative damage, etc.) appear to have been overwhelmed in the immune cells in these diseases. That’s not such a surprise given what we know about oxidative stress and ME/CFS.
Mitochondrial Calcium
Increased levels of mitochondrial calcium – which can drive the production of reactive oxygen species (ROS) in the mitochondria – provided another potential explanation for the increased levels of oxidative stress. Indeed, mitochondrial calcium levels were highly associated with low antioxidant levels (SOD2) in both ME/CFS and long COVID.
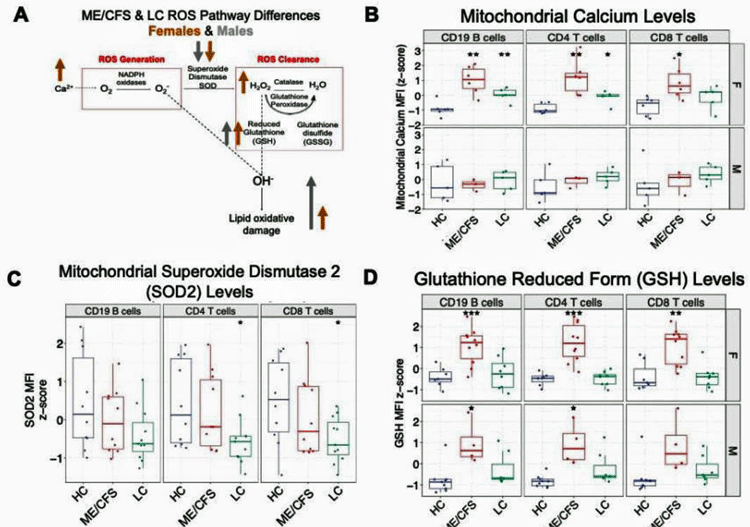
From the study. Check out the dramatically elevated mitochondrial calcium levels in the ME/CFS patients (red), in particular (healthy controls – blue, long COVID – green). Also notice the reduction in the SOD2 antioxidant but the dramatic elevation in the ME/CFS patients’ glutathione levels – indicating that an up-ramping of the antioxidant system has occurred.
That was a very interesting finding given Wirth’s and Scheibenbogen’s 2021 hypothesis that calcium overload in the mitochondria could explain ME/CFS.
“The consequences of that were potentially vast. The high sodium levels turned the tables on the calcium pump, causing calcium to be imported into the cell instead of getting exported out of it. That calcium overload then impaired the mitochondria and other metabolic parts of the cell from working properly and with that – bam! – a new hypothesis (calcium overload) explaining the fatigue, post-exertional malaise, and inability to exercise, think well, etc. was born.”
Klaus Wirth’s Mitodicure startup now has a nice website. Wirth believes a drug called MDC002 will be able to stop the mitochondrial calcium buildup found in the skeletal muscles in people with ME/CFS and similar fatiguing diseases.
Metabolomics
The study also took a deep dive into metabolomics, which assesses the metabolic activity of the cells. The difference between this study and others – which looked at a broad array of cells – was its precision: this part of the study was focused entirely on T-cells.
Would the metabolic activity of T-cells mirror the metabolic activity of other cells? It did – suggesting a systemic metabolic problem may be present. Perhaps to no one’s surprise (at this point) problems with lipid metabolism popped out. Lipids are fatty compounds that provide a dense energy source for our cells, protect our cells, regulate the production of hormones, help with signaling, etc.
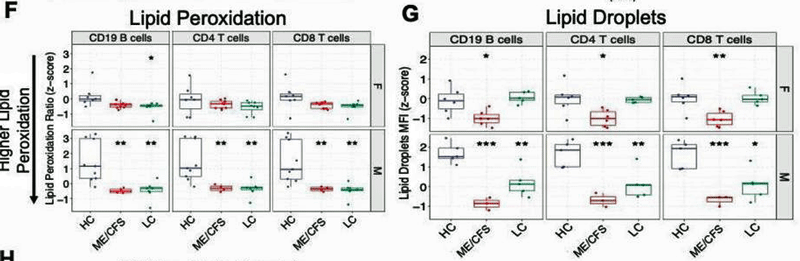
From the study. Note the increased lipid peroxidation in ME/CFS and long-COVID patients (red/green) (lower score = more lipid peroxidation) and dramatically reduced levels of lipid droplets which mop up damaged lipids in the ME/CFS group, in particular. The authors proposed that long COVID is an intermediate form of ME/CFS.
THE GIST
- Health Rising reported some time ago becoming obsessed with the energy problems found in ME/CFS. Vishnu Shankar, a PhD. Stanford student, engineered a study that assessed energy production in ME/CFS patients’ immune cells. The study recently appeared in preprint form and includes some new findings – so it’s onto round 2 of this fascinating study.
- Shankar focused on immune cells because they provide a good test case for assessing energy production. It turns out that protecting the body from pathogens turns is quite an energy-intensive project. Even when it’s not fighting off an infection, the immune system uses about 15-20% of our energy.
- The study assessed both mitochondrial activity and oxidative stress in immune cells (PBMCs) in ME/CFS patients, long-COVID patients, and healthy controls.
- The immune cells in the ME/CFS/long-COVID patients weren’t pumping out as much energy as the healthy controls’ cells – suggesting they were damaged and possibly exhausted.
- Because damaged mitochondria can become free radical-producing machines, they took a look at oxidative stress (free radical) levels. (Just as a damaged automobile engine produces more exhaust than a well-functioning one, damaged mitochondria spew out more toxins; i.e. free radicals.)
- Immune cells have to switch their after-burners on to get the energy to go after pathogens. Producing that energy comes at a cost, though, in the form of increased levels of reactive oxygen species oxidative stress (free radicals). (Note the key word – reactive oxygen species – these are unbalanced oxygen molecules which try to achieve balance by ripping electrons from other molecules; hence the word “reactive”.)
- Dramatically higher levels of reactive oxygen species were found in both the ME/CFS and long-COVID patients’ immune cells compared to the healthy controls. (ME/CFS patients had the highest levels). A closer look revealed, however, that the reactive oxygen species were almost wholly centered in the immune cells from female ME/CFS patients.
- Both male ME/CFS and long-COVID patients did, however, exhibit elevated glutathione levels – indicating that their immune cells were also dealing with increased oxidative stress levels – which have triggered the production of the master antioxidant in our cells – glutathione.
- Altogether, multiple pathways that deal with oxidative stress (glutathione, superoxide dismutase, lipid oxidative damage, etc.) appear to have been overwhelmed in the immune cells in both ME/CFS and long-COVID patients. In all cases, the ME/CFS patients were worse off than the long-COVID patients – leading the authors to suggest that long COVID was an intermediate condition between health and ME/CFS.
- Increased levels of mitochondrial calcium – which drive the production of reactive oxygen species (ROS) in the mitochondria – provided another potential explanation for the increased levels of oxidative stress. That was a very interesting finding given that in 2021 Wirth and Scheibenbogen proposed that increased mitochondrial levels of calcium were a core factor in ME/CFS.
- Once again, damage to the lipids that protect our cells and the organelles in our cells popped out during a metabolomic analysis (of T-cells). Since reactive oxygen species target lipids this finding make sense and it underscored what big deal lipid issues have become in ME/CFS studies over the past few years.
- Once again, men and women had different findings. Oxidative stress was worse in women while lipid damage was more extensive in men. Plus, the study suggested that when confronted with high oxidative stress levels, women’s T-cells go on a hyper proliferation binge – potentially sucking more energy from the rest of the body.
- Trying to reduce the oxidative stress present, they used antioxidants like NAC (increase glutathione), metformin (increase SOD2 expression), and liproxstatin-1 (reduce lipid peroxide levels) in cell cultures and found that NAC and metformin was able to reduce immune activity to some extent. That finding suggested that the right antioxidants might be able to tame the immune activity and improve energy levels.
- All in all, the authors proposed that long periods of elevated reactive oxygen species damage the mitochondria, producing a long-term problem of energy depletion. Because the immune cells already use up so much energy in the body, and then struggle to produce energy in ME/CFS and long COVID, the authors proposed that immune problems in these diseases produce an energy sink that draws energy from other areas of the body.
Next, they assessed “lipid droplets” which are: a) formed to mop up damaged lipids; and b) contribute to acetylcarnitine metabolism – an important part of the energy production. Substantially lower levels of lipid droplets suggested that: a) damaged lipids were not being taken up; and b) that another source of fatty acid metabolism was wanting in the ME/CFS and long-COVID cohorts. Interestingly, while women showed more evidence of oxidative stress, men showed more evidence of lipid damage.
Once again, the ME/CFS patients appeared to be worse off. The findings suggested that long-COVID patients are in an intermediate state between healthy controls and people with ME/CFS.
Gender Gap Explained?
The finding of greatly increased oxidative stress levels in the women’s immune cells was intriguing given the gender gap (predominantly female) found in these diseases. This study found that as the levels of reactive oxidative species increase, the T-cells in women with ME/CFS go on a proliferation binge that requires 10 x’s as much energy to maintain.
They made a stab at explaining why the gender gap exists. Not surprisingly, it may all come down to sex hormones. It turns out that sex hormones like estradiol regulate the levels of the enzymes (catalase, glutathione peroxidase, SOD2) that trigger antioxidant production. That suggests that reduced estradiol levels in women could predispose them to reduced antioxidant production.
Indeed, a study that used routine lab tests to follow people after a bout of infectious mononucleosis found reduced estradiol levels in the still-sick patients 12 and 24 months after the infection. Estradiol levels recovered after that, but that two-year period of reduced levels may have been enough. The Shankar paper predicted that it might only be a matter of time before the mitochondria in our cells become too battered to return to health.
Treatment
Now that they’d found this oxidative stress/energy production problem in immune cells, they tried to turn it around using antioxidants like NAC (increase glutathione), metformin (increase SOD2 expression), and liproxstatin-1 (reduce lipid peroxide levels) in cell cultures.
The small laboratory trial (5 ME/CFS females/6 healthy controls) found that NAC was able to significantly reduce the T-cell hyperproliferation by about 10%, and metformin by about 6%, but had no effect on healthy controls.
Whether that’s sufficient or not is unclear, but the authors considered that oxidative stress constitutes a “tunable molecular link” that could be controlling the energy-draining T-cell proliferation found: i.e. reducing oxidative stress might be able to “re-tune” the immune system activation, allowing it to return to normal.
The authors proposed that assessing oxidative stress levels in immune cells – apparently not a difficult test to do – could identify people who might benefit from this approach.
The Hypothesis
In the end, the hypothesis goes like this: As the immune cells take their foot on the gas to fight off an infection, levels of reactive oxygen species (ROS) (free radicals) explode. For whatever reason, the inability to rapidly clear the virus leaves these reactive oxygen species elevated for long periods of time, causing damage to the mitochondria.

The authors proposed that the immune system’s flailing about to get more energy is draining energy from the rest of the body. (Edgar Allan Poe, Public domain, via Wikimedia Commons)
The immune system – flailing around for more energy – becomes an energy sink, drawing energy from other parts of the body.
The authors believed the metabolic changes they found “likely” extend to the entire immune system, and “if systemic, could explain the prevalence of fatigue and other symptoms in these two syndromes”. To put it in plainer terms, they propose that the high levels of oxidative stress and inability of the immune cells to produce proper amounts of energy could explain everything.
Demonstrating the importance that oxidative stress may play in ME/CFS, the study was able, for the first time, the authors said, to directly link increased levels of oxidative stress with reduced mitochondrial functioning in both ME/CFS and long-COVID patients.
A second major takeaway was that the study was able to show the increased oxidative stress particularly affects immune cell activation in ME/CFS/long COVID. Since high levels of oxidative stress appear to cause the T-cells in females to hyperproliferate – which, in turn, potentially produces an energy drag – finding ways to turn down the oxidative stress levels could conceivably help with energy levels.
Moreover, it suggests that at least in females, ROS levels may serve as a tunable link for adjusting T cell proliferation.
Conclusion
Perhaps the most significant outcome of this study was to see a now rather familiar array of themes pop up. The fact that oxidative stress, mitochondrial problems, lipids, fatty acid metabolism, female hormones and gender all showed up suggests the field is getting at some core issues in ME/CFS and long COVID. The increased mitochondrial calcium levels were particularly intriguing given the key role Wirth and Scheibenbogen predicted they play in ME/CFS in 2021, and Wirth has found a drug that he thinks could help. The possibility that relieving oxidative stress could tamp down some of the immune activation found in this illness was intriguing. The question, of course, is how to do that.
While the study opened up some interesting possibilities, it needs to be validated and expanded. The fact that it grew out of a big NIH grant may mean it has a better shot at getting a nice, fat NIH grant that is able to determine if a systemic energy drain is present in the immune system, if it’s affecting the rest of the body, and the best way to address the mitochondrial/oxidative stress issues found.






Dear health rising
In all the great research and theories i have read on this
Site , i have never seen any mention of possible enviromental factors that could be either be adding to , or causing long covid me/cfs.
Microwaves are a very real threat to public health whether it be wifi or mobile telecommunications.
5g is a factor of about 1000 times more powerfull than 4g and needs a much more localised booster network , approx every 100 metres due to its tiny wavelength ( millimetre wave)
These telecom microwaves are having a huge impact on birds and bees built in navigation systems.
They increase oxidative stress considerably.
They also seem to effect cellular calcium levels.
Please find some links below
https://www.sciencedirect.com/science/article/pii/S0013935118300355
https://pubmed.ncbi.nlm.nih.gov/34778597/
I found that interesting n the science direct article as well, the calcium connection.
thanks for sharing.
“• …Ca2+ rise.
• Wi-Fi is thought to act via voltage-gated calcium channel activation.”
I’ve been taking NAC 900mg for a few years and while it does give me such an ever so slight boost it has not been enough for my health to improve. Can’t wait to see some new drug options!
Interesting about the calcium connection.
I’ve just gone through a cancer scare: my calcium levels, which have been borderline high for years (decades? since 1989 when I got sick?) have suddenly be declared ‘other side of the border’ (because the ‘normal’ level was lowered a bit, not because it changed), and my GP got all nervous and had my parathyroid checked, and got levels of kappa and lambda light chains which are apparently suspicious, and sent me an oncologist referral.
Who had her ask me to do one extra test before I went (for immunofixation to determine if it could be a monoclonal gammopathy which might indicate multiple myeloma, a cancer of the blood).
Result: “No overt evidence of a monoclonal protein.” And the oncologist doesn’t want to see me!
All this from slightly elevated calcium levels because THEY changed the ‘normal range’ numbers.
But I’m interested in this idea that the calcium level is more important, and the idea that something is eating up all our energy is an obvious possibility (rather than us manufacturing less energy). Will be looking forward to hearing more about this.
And I don’t know exactly how long my calcium levels have been just at the normal range upper level, but will check whatever medical records I have from BEFORE 1989 with AFTER 1989.
@Alicia, I see your doctor is worried that you might be in the realm of MGUS–a precursor of multiple myeloma and some other blood/plasma cancers. I recently found out I have MGUS. It’s very complicated and even though you don’t fit the Dx, I hope your doctor is up date in this arena. If you want to educate yourself more, http://www.healthtree.org is a great place to do it. They have classes, mentors, and a way to track your labs. Precursors to these disorders can take decades to develop and let’s hope this is true for you! I also worry that my ME/CFS symptoms might be connected to my MGUS status as there is so much overlap–it’s hard to sort them out. Best wishes to you and try not to worry too much.
The approach and results of this study smell of conspicuously robust scientific method. And there is Ron Davis in the author accreditation – no surprises. Thank you again Prof Davis for keeping the fires of genuine progress burning.
Head-spinning info, and a lot of it. I get the feeling this one must have taken a lot of time. Thanks for helping us out, Cort.
Many thanks to Dr Shankar and colleagues for their hard work. Good to see research by different teams beginning to mesh.
And thanks to Cort for great coverage as always.
I think I’m finally getting the mitochondria – free radical connection! I’ve heard the terms, of course, repeatedly through the years, but never really understood the connection before.
Thank you!
Mitochondria, mitochondria, mitochondria!
I believe mitochondrial dysfunction will be discovered in any cells/tissue scientists care to test.
It’s a mitochondrial disease. Possibly autoimmune in origin given the speed with which some individuals are affected.
I went from completely normal to severe ME in the span of a day. I can name the day down to the hour when my symptoms began. It hit me like a ton of bricks with extreme muscle weakness, severe muscle pain, extreme fatigue and complete inability to sleep. A cascade of other symptoms followed.
I don’t think the energy deficit in the immune cells causes ME, rather it’s a result of ME. The mitochondria in every cell are dysfunctional. Cells with greater energy requirements (muscle, immune cells, brain, GI) will display greater symptoms.
Anyway, that’s my n=1 opinion from my sick bed.
KH,
can you also tell us what happened right before your ME symptoms started? Were you sick (still sick)? Had you being wirking too hard for too long or perhaps having to deal with a stress overload for an extended period?
Virus? Bacterial infection? Just left hospital?
Thank you
I can pinpoint the start of my severe ME to the morning of 13th May 2012. I woke up with all symptoms… or most… have worsened, never worked again. I was mid a horrible virus, but no idea which as in the UK we do not routinely test. I was frightened and knew something highly abnormal had happened to me. The GP came to me and after bloods, lungs, brain and spine MRI all tested, ME was diagnosed. I’ve had many more tests since…. also medicated for OI and migraine.
Jane,
thank you for your openness about that. That is heavy. I find it vety helpful to know, however.
If I may, I would like to clarify:
you were already in the hospital with the virus when this occurred?
Had you been given antibiotics already?
Last question: what does OI stand for in this case?
Thank you so much. Chris
Not in hospital, just very ill in bed. The virus disappeared after about 10 days…. we rarely go to the GP as viruses don’t respond to antibiotics so it’s symptom management and I thought it was just bad flu.
OI is orthostatic intolerance…. my BP doesn’t pump blood up fast enough after lying down and I’m unable to stand up for long. It’s diagnosed by a cardiologist in the UK with a tilt table test and I’m prescribed Fludrocortisone, Midodine, compression garments and extra water. Some people also have POTs… tachhycardia on standing.
In late December 2017 I got what I thought was the flu. I was sicker than usual for me, but recovered normally within a week.
Late January 2018 I got another virus. Most likely the flu (it was going around a lot at that time). I was quite ill with high fevers for 3 days, then recovered.
February 2018, another virus! This time a head cold/sinus one. 2 weeks later a GI event that lasted several days.
By March I was pretty wiped out and went to my Dr because I was sure something was wrong with my immune system. Historically, I would rarely get sick at all, let alone 4 times in 2-3 months. I had called off of work more times in those 2 months than in the previous 20 years combined!
Dr ran some basic tests and said all was normal (insert your shocked face here) and told me that the flu season had been rough and a lot of her patients had reported multiple viruses similar to me.
By mid-March my energy returned and I went back to my normal life. Working full time, riding my horse 4 days a week, hanging out with my friends, traveling, etc.
On June 3rd at 12:45 pm I was taking a horseback riding lesson when my legs just stopped responding to my commands. I thought it was just a really strenuous lesson and stopped for a break.
My instructor could see my legs trembling from 20 meters away! No pain, just very weak. We decided to call it quits and I went home.
A few hours later the pain set in. 6 years later and it has never stopped. By the next day I was only able to walk 3 steps, too weak to raise my hands above my shoulders, couldn’t stand long enough to brush my teeth, 8/10 muscle pain, muscle cramps, muscle fasciculations, unable to sleep AT ALL. Completely debilitated in the span of a day!
Went back to the Dr, she ran many, many tests. Only abnormality was an elevated ANA. After that came the years of searching for a diagnosis with specialists all over the country. (I’m sure you are familiar with this story).
Many more symptoms followed as the disease progressed and a slow recovery began.
Finally diagnosed myself with ME off the internet in the Fall of 2022 and got an appointment at INIM for confirmation.
I am currently moderate. Back to work part-time. No longer able to ride my horse or do most of the things I used to do prior to 6/3/18.
Please consider reading about / listening to Dr. Terry Wahls who developed her Wahls protocol diet and supplements and went from only being able to take 3 steps to riding her bicycle to work one year later and resuming her hospital rounds.
She healed her own Multiple Sclerosis and is now doing clinical trials with her modified paleo diet on patients.
Your sudden loss of activity and especially the quote “3 steps” reminded me of her.
Her focus is on reversing autoimmune diseases through her protocol, and MS is central for her, but she speaks of many autoimmunities.
We didnt have wifi in 1993 when my immune system decided to ruin my life, but,
At a time when I was still trying to hold my job down, and sleeping in a tent in my back yard,….I would leave the tent in my back yard in the morning and head into the house for a quick shower before leaving for work….
I would enter my home and within a few seconds I would break out into a total head to toe body rash…not only a rash but I also felt my body and overall health decline just as fast as the rash,and along with the rash.
I’ve always suspected it to be electricity.
My suspicious was verified when I went out to my brother’s place of employment and entered a high voltage room with several electrical components mounted on all 4 walls. I began to staggar like a drunk and had to leave or who knows what would have happened.
These cell towers have to have some negative effects.
Place your cell phone into a metal box and tell me if it rings
please, please, please…when will we see larger numbers of patients and controls in the studys? they are so so tiny that i ask myself if they even are statisticly significant… when i did statistics, it needed to be larger numbers… just no money for us…
The study that Cort is presenting here is a so called prospective study. They are typically run with 5-30 probes of patients. And their aim is to find patterns that are shared in all examples in order to develop a hypothesis of what causes ME/CFS. As soon as you have managed to find a good hypothesis that is supported by a prospective study you can think about how to design a bigger study and what you want to find out with it.
In ME/CFS the mitochondria are at the center of interest for a long time but no one has yet been able to formulate a clear hypothesis that would explain how and why a problem with the mitochondria should be understood as the cause of the disease. Therefore we have only prospective studies in this area.
There is even a group of scientist who claim that the mitochondria are not at the root of ME/CFS but that we have to look elsewhere and that the mitochondria are afflicted by something that is transported to them through the blood. Here is a summary of the research into the mitochondria:
https://meassociation.org.uk/2019/07/mea-summary-review-the-role-of-mitochondria-in-me-cfs-13-july-2019/
The study presented here is interesting because it looks specifically at the mitochondria of the immune cells. In other recent studies (Maureen Hanson) it was found out that especially a certain type of T-killer-cells were “exhausted” in ME/CFS.
However, I am not convinced that it is just the exhaustion of the mitochondria of the immune system that causes the debilitating fatigue during and after an acute exacerbation of symptoms.
I think that Vishnu Shankar and others should continue to look at the immune cells but should go a step back and brainstorm about other problems that could affect them and bring stress to the whole cell not just the mitochondria. I am not a scientist – the only field that I consider myself an expert is ME/CFS research. Therefore I can’t formulate other questions about what could go wrong in the immune cells in ME/CFS.
What I can say is that I would have a closer look at whether there was some EBV reactivation smoldering reactivation in B-cells in early ME/CFS and exercise intolerance Long Covid. Because it shows up constantly in all the studies in LC-patients who have exercise intolerance / PEM (Prusty, Iwasaki and more) I would go in this direction. Because EBV is a virus that is known to be able to manipulate immune cells in order to replicate. B-cells are mainly affected. EBV is also known to rarely trigger auto-immune reactions that can be lethal.
thanks for explaining but is it not normal that i, we want to see many really big studdys instead of this? I know, you begin somewhere but waiting and declining for 30 years is a bit to long. And still no huge research studys! This one can be done to… Worldwide. the biggest studdy i know is the DecodeME study working together with precisionlife… That is 1 study for all the suffering around the world for ME/cfs
No, I don’t want to see big studies. I want to see studies by researchers who acknowledge what has already been done, studied and found out about ME/CFS in the area of their research question and then formulate clear and simple questions in their study that can be brought to an empirical test.
The most important thing is to find out about the pathomechanism of ME/CFS. Therefore I would like to see the research money go to the leading researchers who are actually following a valid hypothesis about the pathomechanism of ME/CFS.
We need everything that has been found out about HHV-6b reactivation in ME/CFS replicated and we desperately need the fields of virology, neurology and infectiology jump on ME/CFS research.
I find that the most important interesting study that was ever done in ME/CFS is a post-mortem brain examination of the brain of a person who had ME/CFS back from the 1990 (!) that could show that the brain tissues were baked in exactly the way that viruses and bacterias are known to damage brain tissues. I once saw it discussed on the website of the ME Association. Unfortunately, right now I can’t reference it.
Another story that comes to mind is of a blood specialist I went to see.
He lived very close to a power station.the wires came directly from the power station,across the river and directly over his home roof. He died very young, I think 40 yrs aprox.
These are the very high voltage wires one would see very near the power source
Dr Wirth is mentioned as having found a drug that can address one of the major problems highlighted here. The problem is there is not warp speed initiated by his company or any other. Why? So many lives are at stake. why dont govts pitch in to help. Dr. Wirth’s company is working on drugs but they say it will take ‘many years’ before they have treatments.
Wirth is trying to raise the money to test the drug. In part because there are no validated endpoints for ME/CFS and no biomarkers it’s difficult to raise money. The validated endpoint problem may (may) be ending – time will tell.
Of course since there’s a huge need out there that only the feds can help out with you’d think they’d be first to help out. Unfortunately, nothing could be further from the truth. They’re largely captured by the big diseases. The Roadmap – which was initiated by NIH officials – did focus on the need for treatment trials. Maybe that will make a difference. Vicky Whittemore did say that some small businesses are applying for grants to do clinical trials. That was encouraging…we shall see!
What about medication for depression? For example: no objective blood tests and measuring points either. Yet there is medication for it.
Yes, there is approved medication for depression. However, it has never been scientifically shown that it actually works. The “evidence” that it does is just anecdotal. Especially psychiatrists have lots of that kind of “knowledge”.
I can recommend to read this interview with Erick Turner:
https://www.madinamerica.com/2023/03/making-a-silk-purse-out-of-a-sows-ear-erick-turner-on-how-bad-research-practices-are-undermining-our-health/
HE’S a psychiatrist who worked at the same time as a clinician and within the FDA and thus realised about 2008 that what psychiatrists believed about the efficacy of so-called anti-depressants wasn’t supported at all by the evidence.
Here is an interview with leading psychiatrist Joanna Moncrieff who has debunked the “serotonin in depression” hypothesis two years ago. She says that there is evidence now that people who don’t go on “anti-depressants” or come off have better recovery rates that people who stay on them.
https://www.newstatesman.com/the-weekend-interview/2023/04/joanna-moncrieff-im-not-convinced-antidepressants-have-any-use
Depression was thought to be caused by serotonin. This is a crazy story. And scientifically unproven. Yet this fairy tale is still told.
Gijs, the fairy tale has two parts. One was the serotonin hypothesis. The other that the drugs actually work. According to the scientific evidence they don’t. It’s literally a cult.
As I said – the evidence is just anecdotal. It doesn’t only hold true for the “classic” SSRIs and earlier generations of so-called anti-depressants. But I am sure the now hyped hallucinogens and ketamin will soon be proved to not substantially support people with chronic depression either.
There are actually critical historians and sociologists who think that since its inception psychiatry hasn’t produced anything but different trends of scientifically-fashioned quackery.
“depression” is a symptom, and not an actual “disease.” and can have so many diff. causes. MAOI’s work very well on “depression,” so you can’t say serotonin and dopamine are irrelevant. In fact it was the scientific community that exaggerated how “dangerous” they were, when they lost the patents and came up with the new SSRI’s. no treatment for depression will ever be that effective bcs there are so many possible causes. It is like saying “what is the perfect job”? it varies for each person…
It’s interesting what your writing, Alexis, however I think it’s pretty confused. What do you actually want to say by “depression is a symptom and not a disease”?
Do you want to say that when you go through a severe depressive episode in the wake of the overwhelm that comes with the severe symptoms and the catastrophic situation around medical care for ME/CFS patients that it shouldn’t be taken seriously and a person shouldn’t try to find help because it is a consequence of social and political problems?
Would you then suggest that social and political problems should fall in the realm of medical research and being medically diagnosed? Would you then agree that doctors should themselves diagnose with a psychological obsession with gaslighting ME/CFS patients and that depression that might occur in the patients for being gaslighted should be looked at as a symptom of said doctor’s disorder?
Or do you believe – as I have seen it in other places lately where people fall for the hype around medical trauma theory – that the event of being gaslighted is a “trauma” and thus a disease and that depression is a symptom of trauma disease? Why do you think that it makes sense to speak of an act of violence and medical abuse acted out by doctor’s as a disease in the patient? Isn’t it rather a problem of these doctor’s whose work is off-base?
Here is an article about the concept of depression in psychiatry: https://www.madinamerica.com/2024/06/the-misrepresentation-of-depression-health-websites-circular-logic-exposed/
and how even the professionals themselves do not understand the concept of psychiaric diagnosis. But you are just adding more to this confusion with your claim that depression is not a disease but a symptom.
Lina, all i am saying is that there is usually a root cause. There are times when the root cause/actual disease is in the brain. And of course, depression is very, very serious. But there is no “golden hypothesis,” hence why the “serotonin hypothesis” is bad. However, even without getting to the root cause, MAOI’s can work very well, at least for a time.
I have experienced about a 10% improvement in fibromyalgia symptoms by taking daily a full dose and a variety of high-quality curcumin supplements. Going whole food plants only 4 years ago was not enough anti-oxidants to get much better. Still experimenting with the brands listed below:
1. Bosmeric-SR Joint Support Supplement, Potent Blend of Turmeric Curcumin with Black Pepper, Boswellia Extract & Ginger
2. Qunol Turmeric Curcumin with Black Pepper & Ginger, 2400mg Turmeric Extract with 95% Curcuminoids
3. Jarrow Formulas Curcumin Phytosome 500 mg
and,
4. Qunol CoQ10 Gummies, CoQ10 100mg
5. Good Natured Premium Spirulina and Chlorella Capsules 1,250 Tablets
Just wanted to share this enlightening conversation between Eric Topol and Svetlana Blitshteyn regarding autonomic dysfunction and ME/CFS and Long-Covid;
https://erictopol.substack.com/p/svetlana-blitshteyn-on-the-front?utm_source=podcast-email%2Csubstack&publication_id=587835&post_id=144790562&utm_campaign=email-play-on-substack&utm_medium=email&r=2ssesd&triedRedirect=true
Cort, maybe you could look into this and comment. Regardless, it is a very interesting read.
This is a very interesting study. However, I still would like to know what the immune system is trying to fight off instead of repeating the idea that it was fighting off a ghost and that therefore we simply need to dampen the immune system from “overreacting”.
It sounds like a reproach that women are used to hear as a criticism when they’re rightly upset about something and trying to set boundaries. Oh, you’re just overreacting we are told. This is mean reaction to women’s anger to shame them of it. I find it upsetting that the immune system is suspected or even claimed to be overreacting when we don’t have any proof whatsoever that an “auto-immune” process like this could be going on.
My current working hypothesis is that we have to look at a form of atypical EBV reactivation in B-cells that we don’t understand yet here. And that that might be the cause of oxidative stress and mitochondrial dysfunction in the immune cells. I think that we’ll find out that ME/CFS is half the cousin of Mollaret meningitis (self-ending, benign HHV-1 brain inflammation) and AIDS (where with every inflammatory episode the immune cells are weaker and fewer because of being the reproductive site of the virus.) And somehow EBV and HHV-6b do this job, maybe together? Or one after another?