

Check out Geoff’s narrations
The GIST
The Blog
(The GIST is about a third way down the page)

An extracellular vesicle called an exosome bearing a heat shock protein. (Image by Guillaume-Pelletier, CC – Wikimedia Commons)
Extracellular vesicles (EVs) have been a relatively new introduction to the chronic fatigue syndrome (ME/CFS) field. They first popped up in 2018 and since then, at least 8 studies have surveyed them in ME/CFS. The Ludovic Giloteaux/Maureen Hanson NIH-funded group has led the way with 4 studies.
Ranging in size from 30-1,000 nanometers, extracellular vesicles are vanishingly small (a human hair is 80,000- 100,000 nanometers wide) packages that are regularly being emitted by our cells. They’re so tiny that it took the development of electron microscopes and ultracentrifuges to find them, but once they were found, they turned out to be everywhere and they pack a punch.
Our cells communicate with the rest of the body by emitting EVs filled with proteins, amino acids, lipids, DNA, and RNA. They can affect many processes in the body including immune and metabolic regulation. Because their composition reflects what’s happening in the moment, studies assess their protein (proteomics) content, gene expression (transcriptomics), etc., to get a snapshot of how the body is responding.
In “Dysregulation of extracellular vesicle protein cargo in female myalgic encephalomyelitis/chronic fatigue syndrome cases and sedentary controls in response to maximal exercise“, Giloteaux, in Maureen Hanson’s group, examined how exercise affects these strange communication devices in ME/CFS.
Their hypothesis was that studying EVs released during/after exercise would help inform what’s happens during exercise and what may be causing the post-exertional malaise people that ME/CFS experience.
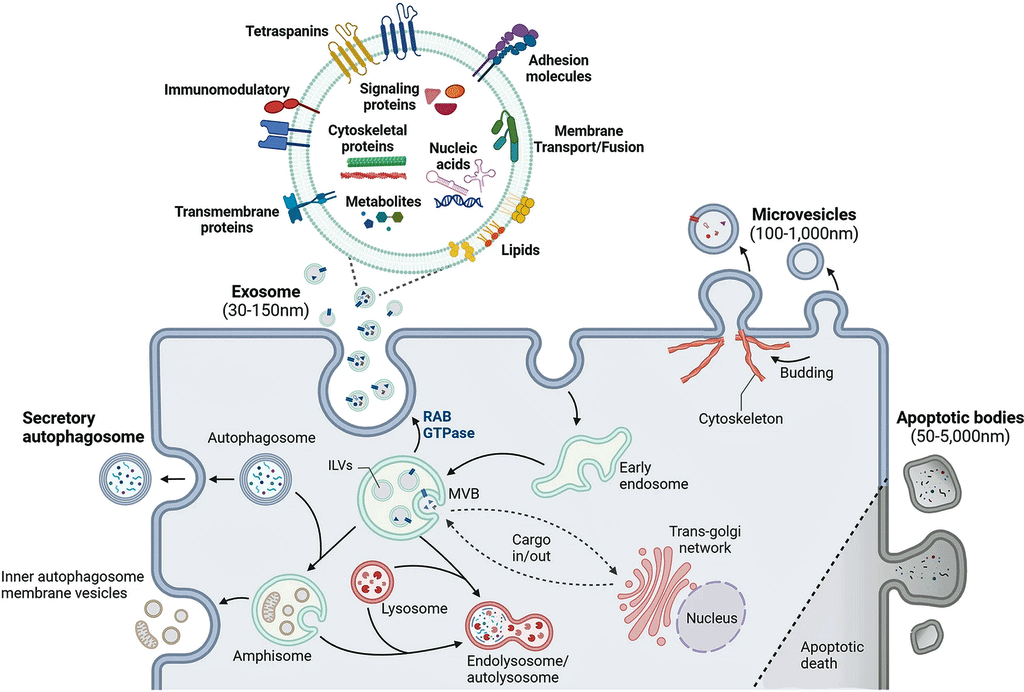
How extracellular vesicles work. (Image by Yu_Jin_Lee_CC_4.0_Wikimedia_Commons)
The GIST
- Our cells communicate with the rest of the body by emitting vanishingly small bags of proteins, amino acids, lipids, DNA, and RNA called extracellular vesicles (EVs). These EVs can affect many processes in the body including immune and metabolic regulation. Because their composition reflects what’s happening in the moment, studies assess their protein (proteomics) content, gene expression (transcriptomics), etc., to get a snapshot of how the body is responding.
- It was no surprise then to see the Gilotreaux / Hanson team at Cornell use them to check out what happens when people with ME/CFS engage in a short bout of intense exercise.
- They found that the EVs in the female ME/CFS patients were “highly disrupted” – and in a familiar way. Just as Hanson has shown has occurred with proteins, gene expression and metabolites, the EVs in the ME/CFS patients simply failed to respond. That is, far fewer EVs in the ME/CFS responded to the exercise than did the healthy controls, and when they responded, they often took longer to respond.
- These findings fit a broad theme that, at the most basic of levels – the molecular level – ME/CFS patients’ bodies simply aren’t responding much to it. It’s as if they’re kind of ignoring that it’s happening at all. When they do respond, their response is also often off – suggesting that they’re responding in a deleterious way.
- Pathway analyses indicated that the coagulation pathways were altered in the ME/CFS patients, which made sense given studies indicating the people with ME/CFS have 10x’s more microclots than normal.
- Because muscles generate force by contracting, anything that disrupts the muscles’ ability to contract is going to impact one’s ability to exercise. Perhaps not surprisingly, a pathway analysis found that EVs involved in muscle contraction were in the top ten most altered pathways shortly after exercise in the people with ME/CFS.
- While proteins involved in muscle contraction increased dramatically in the healthy controls after exercise, they were either reduced or did not increase in ME/CFS. Similarly, EVs associated with muscle-related tissue enrichment increased in the healthy controls but failed to do so in the people with ME/CFS.
- Rob Wust’s recent long-COVID muscle biopsy exercise study appears to back up some of these findings. Wust found frequently damaged, and even dying, muscle tissue after exercise (in some patients), and an increased emphasis on the dirty and inefficient anaerobic energy production system.
- A protein called clusterin (CLU) really stuck out for the researchers. It was the only protein that was both positively correlated with the degree of fatigue pre-exercise and with the amount of muscle and joint pain after exercise. It also decreased in abundance after exercise in the healthy controls but not in people with ME/CFS.
- Clusterin is involved in several processes that other studies suggest may be impaired in ME/CFS. It clears out cellular debris, helps remove old and damaged cells, and helps fold protein correctly. Clusterin levels were associated with increased muscle and joint pain and fatigue. The authors suggested that “this protein may have an important role in ME/CFS pathophysiology.”
- Suggesting perhaps that a herpesvirus had reactivated, the researchers found evidence of upregulated immune pathways that respond to “antigen presentation”; i.e. the appearance of a pathogen or other substance that tweaks the immune system.
- While nobody knows what is causing the strange lack of a molecular response to exercise, the fact that it’s been demonstrated to happen in some different compartments (gene expression, EVs, proteins, metabolites) in different studies suggests the group is on the right track.
The Study
The NIH-funded study involved 18 females with ME/CFS and 17 age and BMI-matched sedentary controls. Blood samples were collected immediately before an exercise test to exhaustion, and then 15 minutes and 24 hours later. We’ve seen exercise affect everything from gene expression, to proteins and lipids at the molecular level. Would that extend to the EVs?
A Failure to Respond
It did. Giloteaux et al. found the ME/CFS patients’ EVs were “highly disrupted” – and in a familiar way – compared to the sedentary but healthy controls (HC’s). The study found:
- Fewer proteins in EVs 15 after exercise in patients compared to controls (Figure 4a).
- Reduced protein expression in EVs after exercise in ME/CFS patients (63 increased EV proteins in ME/CFS vs 178 E in HCs).
- Delayed response – a delayed increase in the abundance of several EV proteins after exercise.
The authors concluded that the ME/CFS patients exhibited a “failure to mount an adequate response to exercise at the molecular level.” The theme was a familiar one. In her exercise studies, Hanson’s group has found the same pattern with regard to proteins, metabolites, and gene expression in ME/CFS patients: for some reason, their systems are simply unable at the molecular level to respond to exercise.
Their systems may be responding in a way that’s injurious as well, but the broad theme is that, at the most basic of levels – the molecular level – ME/CFS patients’ bodies simply aren’t responding much to it. It’s as if they’re kind of ignoring that it’s happening at all. Given how stressful exercise is, that could explain a lot. One wonders how much problems with energy production at a systemic level could play a role in that.
Check out some reports from earlier blogs on Hanson’s team’s findings.
- Gene expression – “As 102 genes in the HC” immune cells exploded into action, the genes in the immune cells of the ME/CFS patients lay low. They basically sat the exercise bout out – no significant changes in gene expression were found.”
- Metabolites – “A small urine metabolomics study that found an explosion in altered metabolites (n=400) in healthy controls but no significant change in ME/CFS patients’ metabolites 24 hours after exercise later.”
- Proteins – “Germain’s study found exercise triggered a much bigger change in the proteins found in the sedentary, but healthy, controls than in the ME/CFS patients. The healthy controls responded to the rigors of the second exercise test by scrambling their protein mix more. Lacking the same ability to do so, the ME/CFS patients did not.”
And now we can add EVs to the mix. Digging deeper into the study, the researchers found:
- More EVs in ME/CFS – Despite the fact that the EVs in the ME/CFS patients didn’t respond as fully to exercise, apparently ME/CFS patients’ cells were shedding them more frequently as more EVs overall were found. The authors didn’t explain why this might be so but did note that higher concentrations of EVs have been found in other conditions, including Alzheimer’s and vascular conditions that affect the brain.
The Crazy Coagulation Cascade
Exercise triggers coagulation in everyone. The coagulation cascade triggered in ME/CFS was, however, quite different.
Looking at the pathways activated or not activated in the EVs, the researchers found that exercise triggered an increase in coagulation activity in both ME/CFS patients and the healthy controls but also that the coagulation pathways found were different in the people with ME/CFS.
Several coagulation cascade proteins (factors VIII and XIII AI, fibronectin) were significantly decreased 15 minutes later in ME/CFS. Likewise, several coagulation factors that have been associated with exercise in healthy humans (FN1, FGA, FGG or FGB) were not increased after exercise in the ME/CFS patients. In a disease characterized by increased levels of microclotting, the reduction in some parts of the coagulation cascade seemed surprising.
On the other hand, factors associated with fibrinogen (FGA, FGB and FGG), which forms the actual clots, were positively correlated with increased fatigue and PEM after exercise in ME/CFS. Likewise, proteins involved in platelets (platelet degranulation, platelet aggregation), as well as wound healing, were strongly correlated with the muscle pain in ME/CFS.
Similarly, increased levels of plasminogen – a new factor which raised a lot of interest in a recent ME/CFS study – were “strongly correlated” with the percentage of time spent reclining or lying down, which itself is, of course, associated with how severe ME/CFS is. The authors proposed that the increased levels of plasminogen reflected an increase in clot busing activity or fibrinolysis. The authors stated:
“The altered temporal profiles of clotting cascade factors in ME/CFS EVs post‐exercise reveals a disruption in the haemostatic balance between clot formation and fibrinolysis.”
That suggested, if I’m reading it right – that clot formation during exercise is being inhibited in ME/CFS (!) – just the opposite of what we might have expected. On the other hand it might reflect the attempts by the body to attack large numbers of, difficult-to-break-down clots. Whatever is happening, the study suggested, as others have, that blood clotting and coagulation are messed up in ME/CFS. Indeed, the authors wrote:
“Other recent reports corroborate the importance of coagulation processes in ME/CFS” – they noted that past studies have found a 10fold increase in microclots in ME/CFS.
Clotting, with all its factors, is a very complex process. Time will tell how EVs affect coagulation in ME/CFS, but this study suggests something has gone awry with that process in ME/CFS and EVs are playing a part in that.
The Muscles
Muscles generate force by contracting. The EVs suggested people with ME/CFS were having problems contracting their muscles properly.
Muscle contraction is kind of the cat’s meow when it comes to exercise. Because muscles generate force by contracting, anything that disrupts the muscles’ ability to contract is going to impact one’s ability to exercise. Perhaps not surprisingly, a pathway analysis found that EVs involved in muscle contraction were in the top ten most altered pathways shortly after exercise in the people with ME/CFS.
Myosin light chain factors help the muscles contract and help repair muscle damage after exercise. Of all the proteins found in the EVs of the healthy controls, they showed the largest increase after exercise. Myosin light chain factors (MYL9/MYL12A/MYL9/MYL16) in people with ME/CFS took a different tack however: they were either reduced or did not increase.
Similarly, while muscle-related tissue/protein enrichment was found after exercise in the healthy controls, no such enrichment was found in the people with ME/CFS. Increased levels of tropomyosin (TPM4), tropomodulin (TMOD3), and calmodulin (CALM2) from baseline to 24 h post‐exercise were strongly correlated with higher levels of muscle pain in ME/CFS.
In the end, the same pattern seen in EVs overall was found in the muscles – a failure to adequately respond to exercise. A decreased or delayed EV muscle response was associated with more symptoms in ME/CFS. While the authors didn’t say so, delayed muscle repair processes might result in more muscle damage/pain after exercise; i.e. PEM.
Note that Rob Wust, in his long COVID muscle biopsy study found that exercise produced similar findings including frequently damaged and even dying muscle tissue (in some patients), and an increased emphasis on the dirty and inefficient anaerobic energy production system. Wust is currently engaged in a similar study in ME/CFS.
Trash Buildup? The Clusterin Factor
A protein called clusterin (CLU) really stuck out for the researchers. The only factor they devoted an entire section of the paper to, clusterin could play a central node in the fatigue/pain problems occurring in ME/CFS. It was the only protein that was both positively correlated with the degree of fatigue pre-exercise and with the amount of muscle and joint pain after exercise. It also decreased in abundance after exercise in the healthy controls but not in people with ME/CFS.
Clusterin is involved in a slew of cleanup processes that other studies suggest could be in play in ME/CFS. It clears out cellular debris, helps remove old and damaged cells, and helps fold protein correctly. In short, something like clusterin seems to make sense in a disease in which unusually shaped amyloid proteins (in the brain and clots) may be present, and problems with autophagy (mitochondrial cleanup) may be occurring. Since intense exercise always produces small injuries in the muscles, cleanup processes probably play an important role in the post-exercise period.
Plus, increased CLU levels have shown up in a variety of neurodegenerative and inflammatory diseases (inflammatory myopathy, rheumatoid arthritis) and have been linked with cognitive issues. It was no wonder that the authors reported:
“In our study, elevated CLU in EVs post‐exercise is associated with worse myalgia, arthralgia, and fatigue indicating that this protein may have an important role in ME/CFS pathophysiology.”
Immune Trouble
The stress of exercise could be reactivating herpesviruses or other pathogens which tend to reactivate during stress. To that end, the researchers found evidence of upregulated immune pathways that respond to “antigen presentation”; i.e. the appearance of a pathogen or other substance that tweaks the immune system.
Conclusion

ME/CFS patients fail to respond normally to exercise at the molecular level. The question is: why?
The Hanson group has done it again: for at least the fourth time, they’ve shown that, at the molecular level, people with ME/CFS are not only not responding normally to exercise but in key ways they simply aren’t responding at all. Whether one is measuring proteins, metabolites, gene expression or EVs, a normal response to exercise is, for some reason, not kicking in during exercise or afterwards.
While nobody knows what is causing that, the fact that it’s been demonstrated to happen in different compartments in different studies suggests the group is on the right track.
The EV study highlighted dysfunctions in some key factors at play in exercise including coagulation, muscle contraction, cleanup or trash removal, and immune issues.
It’s great to see the exertion problems in ME/CFS validated at a molecular level. The important next step would presumably involve trying to understand why, when the stress of exercise appears, ME/CFS systems are unable to respond normally.
The NIH’s Molecular Transducers of Physical Activity in Humans Program (MoTrPAC; Motor…Pac – get it? Kind of?) might provide some clues. The 2016 MoTrPac Initiative was designed to track “exercise’s impact on biological molecules” which, if I’m not mistaken, Hanson’s studies just did. Two the 19 original grants went to two Stanford researchers (Michael Snyder, Stephen Montgomery) to identify and characterize all the molecules that form during or after exercise, and Snyder has been involved in ME/CFS research.
Time will tell but Hanson’s group has got us off to a great start.






I was just talking to a friend the other day who is a personal trainer. He used a handy acronym SAID: Specific Adaptation to Imposed Demand. I told him that is exactly what is severely impaired in ME/CFS And this great article gets us all closer to understanding the mechanics of that impairment.
During CPET my body has to go into overdrive in stead I’m going into hibernation.
Appelmans Wüst might want to have a peak at my (30+ years) of muscle damage.
All those years my muscles ran “on empty”
If you look back quite a while now to Naviaux’s Dauer and Lemle’s hibernation hypothesis these findings seem quite striking indeed!
With Mark Davis’ theory, the recent discovery of direct inflammation/immune control by the brain and Nath’s findings of “effort preference”, some type of centrally enforced energy-saving mode might be going on. My wild guess is, the brain is trying to balance energy expenditure between clearing a chronic infection (or several infections) and other body needs. CFS with PEM that happen after a toxic exposure might be because the toxins damage the immune system in some way which then triggers some backup immune mechanisms to control persistent pathogens that are more energetically expensive.
I must have paid attention to your blogs Cort, thanks for doing the hard work!
Have you tried your resistance training lying down?
No orthostatic intolerance to combat; could that reduce PEM?
I don’t know if men show the same abnormalities, but we can conclude that exercise and ME do not go together. It’s a big mess of disruptions. Many of our disturbances go against all medical laws of nature, it seems. This is a special and nasty disease isn’t ?
I was encouraged to read this statement in the paper. “Suggesting perhaps that a herpesvirus had reactivated, the researchers found evidence of upregulated immune pathways that respond to “antigen presentation”; i.e. the appearance of a pathogen or other substance that tweaks the immune system.”
I believe the herpesvirus of most concern is HHV6 A. A review paper published in 2023 gave a good overview of HHV6. Originally called HBLV , HHV6 was discovered in the AIDS lab in 1986 and subsequently divided into HHV6 A & B.
This excerpt from the paper explains this.
“Human herpesvirus 6 (HHV-6) was initially discovered in blood lymphocytes of adults with lymphoproliferative diseases or AIDS and was labeled human B-lymphotropic virus. Further research identified HHV-6 in CD4+ lymphocytes and as a member of the herpesviruses. As it was the sixth herpesvirus isolated, it was then subsequently renamed human herpesvirus 6. Typical of a herpes virus, HHV-6 has been known to establish acute, incessant and permanent infection.
HHV-6 is the collective name for the double-stranded DNA viruses HHV-6A and HHV-6B. HHV-6A and HHV-6B, are officially recognized as distinct viruses instead of variants within the herpesvirus family. While much less is known about HHV-6A, it occurs more frequently in the immunocompromised host. In contrast, research has identified HHV-6B as the etiologic agent of the childhood illness exanthema subitem (roseola infantum).[1] The acquisition of HHV-6B is quite common in the young and is frequently seen throughout emergency departments worldwide. HHV-6B is a ubiquitous virus, with over 90% of the human population infected within the first 3 years of life.”
Testing for HHV6 A is only available at specialty labs associated with the HHV6 Foundation and is quite costly so we have no idea of the prevalence of this virus that is found more frequently in immuno-compromised hosts.
Betty – I definitely agree that the herpesviruses are suspect. Given the ubiquity of the herpesviruses, and the fact that most people seem to be either not affected or at least able to recover sufficiently, I strongly suspect that there is an underlying genetic anomaly, probably sex-linked, that determines whether an individual develops ME/CFS. (My CFS began with first an EBV infection, and then a massive CMV infection.) The fact that – finally, after all these decades – researchers are now realizing that they have to look at the sexes separately, gives me a small amount of hope. I’ve been waiting 40 years for that!
Hi Judith, I believe you may be right about genetic predisposition and I also question prior environmental exposures. I was exposed to Dursban for flea control in our home right before I became ill. I also was born and grew up for the first six years in Mississippi at a time when they were changing from manual to chemical farming. Several relatives died of chemically-related diseases and I had the worst cases of all the childhood illnesses and terrible allergies. Even before I was exposed to Dursban, when I would go in Woolworth’s dime store if they had sprayed with pesticides, my thumb or upper lip would get numb.
My brother, who was three years younger, and grew up in the same environment just died of acute leukemia.
In the work I do, we believe that the mother or father’s exposures can affect the developing immune system of the baby leaving the child vulnerable to illnesses like ME/CFS, autoimmune problems, cancers and neurodevelopmental problems.
I love the Hanson group’s work. Didn”t fibronectin also show up in Prusty’s hypothesis?
Still think that someone (you? HRP? the both if you? :-)) should do a review of all exercise intolerance related findings in ME/CFS as a publication. Question is, before or after the NIH intramural study will publish their PEM findings?
I meant to reply to Cort. Betty, I always find your posts really interesting. Thanks for sharing your background history regarding chemical exposure. I am sorry how your family was affected.
Ha! Check out this study!
I was shocked to see someone doing this. A 2-day exercise study of people with ME/CFS triggered by a toxic exposure gets the same results as other 2-day studies.
“This is the first study to conduct a 2-day consecutive CPET in previously exposed HD participants (toxic exposure) with CFS symptoms. Our results confirm previous work that demonstrated abnormal responses to PEM in CFS patients. ”
https://pubmed.ncbi.nlm.nih.gov/36916046/
“Although fatal lung damage was initially reported, further investigations revealed that the damage was not limited to the lungs; systemic damage also occurred [3,4]. Among individuals with exposure to HDs [humidifier disinfectants], although a lung disease was not evident, some complained of chronic fatigue and post-exertional malaise (PEM) compared to healthy individuals.”
The author provides no references that link HDs and ME/CFS. None of the sources cited in the paragraph discussing the supposed link even mention CFS or PEM.
They didn’t even recruit based on if the CFS symptoms came after HD exposure, as far as I can tell:
“Twenty-nine participants with a history of HD exposure and reported CFS symptoms were recruited from specialist clinics and support groups in South Korea.”
I first heard this theory, including that it was probably HHV A, from Dr. Paul Cheney in 2009. I think he would be pleased, but not surprised, to know that he was on the right track.
Correction: HHV6 A
Me/cfs is just a huge tangled knot of physiological abnormalities. It seems the only way forward would be to identify the main upstream driver(s) and target those. But as of yet, I don’t sense that anyone is closing in. Just endless discoveries of downstream abnormalities..which gets us nowhere in terms of treatment.
Hi Linda and everyone commenting on this blog. As far as I know, Dr. Cheney saw more ME/CFS patients (thousands from all over the world) than any other doctor. He was my doctor for more than 20 years and helped me achieve what he termed a “functional cure” until I got Covid the first time. Notice, he didn’t say cure because you are never cured of a herpes virus. You can only hope to keep it suppressed.
You have to ask yourself the question: if the most experienced doctor in this field thought ME/CFS was caused by HHV6 A why hasn’t a large study been done looking at this virus? Why isn’t a test for this virus which by the way can be measured in hair follicles, nail clippings and menstrual fluid been offered to the public?
I will tell you why. It is called “don’t panic the public”. I have a lot of experience with this. Our son was born with a missing hand caused by a medication used for morning sickness. I didn’t figure this out on my own. Whistleblowers from the FDA told us to pursue it. Eventually a national news program did a three month investigation and filmed a story. It was never aired because the FDA and CDC sent them letters to “not panic the public”. In the meantime, more children were born with missing limbs. It took us 10 years, but we eventually got this drug off the worldwide market.
Establishing a cause of ME/CFS means that insurance companies, Medicare and disability providers have to cover the cost and care of patients.
As long as this is left to “the more study is needed industry”, we will only get descriptions of symptoms, not a cause or a cure.
If Dr.Cheney’s treatments provided me with a functional cure, they might be refined to offer more permanent suppression of HHV6 A.
Nancy Klimas has found, I believe, that HHV-6 is the most commonly reactivated virus in ME/CFS.
I meant to reply to Cort in case you were wondering about the random comment. (Unfortunately, my brain seems to have mixed up replies – first replied to you instead of Cort, then to myself instead of you – see below)). Thank you for your interesting comment!
Yay, More sh*t is wrong with us. How about someone try figuring out why?
I think we got to one why: the body is simply not responding to exercise. It’s not simply an aberrant response to exercise – in substantial part it’s a non-response to exercise.
I’m comforted by the fact that these studies agree that a major part of the problem is a failure to the body to simply respond to exercise. I don’t think that was on anyone’s radar a few years ago. That realization should I think lead to more questions why something like that would happen?
It reminds me of Naviaux’s Dauer hypothesis, Nath’s findings suggesting the brain is shutting down operations, systemic mitochondrial breakdown, that is depleting the tissues everywhere of the energy they need.
Hopefully, these findings are a step on the way to the bigger why.
Cort – Thank you for all you do! The importance of your updating us regularly, synthesizing studies and new info. into laymen’s terms, spreading HOPE and LIGHT among us and your long standing commitment the site you have built helps many of us counter some of the burden this illness(s) has brought us. BLESS YOU! ☀️
I have 2 kind of dopey questions. I was bed bound for 2-3 years. I am not anymore for many years. I now hike carefully, pace myself, rest when I need to. But, I can’t seem to build muscle mass. In fact it seems to be decreasing all over my body. If I worked out carefully and slowly with low weights and worked up to heavier ones would I still be able to build muscle mass at all? Would I at least reap SOME benefits of a healthier body? Or is it just going to do more damage than good? I have had this for almost 40 Yrs. My brain is too fatigued and scattered to understand the above info well.
Also, how high is too high for lipids to be in ME/CFS with 10X higher incidence of microclots? My dad had a triple bypass and my mom died age 31 from a pulmonary embolism that travelled from leg to lungs causing sudden cardiac arrest. My GP doesn’t think my high lipids matter. Where could I find info to take her specifically showing it matters in ME/CFS? Or am I interpreting this info. wrong? Thank you! Sorry for my brain fog and wordiness.
My feeling personally is that if I can get exercise in whatever way I can I will do it. The more I look at longevity stuff the clearer it is that exercise is very important.
If you can do it without untoward side effects I don’t see why it wouldn’t help. I do find that I get stronger using exercise bands – I can go longer over time – so something good is happening. Often I will have some PEM afterwards but on the whole, personally, I think given the negative effects of no exercise that doing whatever you can is good.
There is a bit of a quandry regarding exercise studies and that is because most people with ME/CFS engage in very little activity any exercise is going produce more damage at the onset.
As to lipids I don’t know but I really interested in finding out.
Hanson’s study, thankfully, accounted for this by using sedentary healthy controls.
Cort, our country is obsessed with the supposed benefits of exercise. My grandmother lived to be 97 and she never exercised other than to push the pedal to the metal to drive from Mississippi up to Memphis to go dress shopping.
My mother lived to be 89 and she never exercised. (My mother-in-law, on the other hand did step classes, jazzercise, etc. She died at 86.) My father lived to be 93 and he never exercised.
My neighbor who is thin, eats organic and exercises every day had a heart attack at 55.
I like to use my cats as a prime example of the ridiculous bunk we have been given about exercise. They may run around for 5 minutes in the morning and sleep the rest of the day. Our senior cat is 15.
Even a cheetah only races to catch prey, but then sleeps the rest of the day.
Please tell me if you think I am wrong. But, I see no evidence from the animal kingdom that random exercise ever happens.
Why then should ME/CFS patients berate themselves because they can’t do weight training or aerobic exercise?
You are so correct….I’ve been working around animals most of my life,also was a taxidermist…not that that matters other than pathogens.
THEY ONLY RUN WHEN NEEDED!!!
and usually short stints
….just to add,…..there is the hunter,and the hunted in the animal world.
I suspect the hunter does way more exercise to catch its prey (the hunted)…but once the hunter gets more skilled at hunting, less exercise is used to catch its prey.
That’s why you see mostly the young hunter being seen in daylight hours rather than at night, because the young hunter isn’t good enough and must hunt both day and night.
Let’s use a coyote for an example.
Being once a trapper many moons ago,i learned a young predator will go days without a kill of a larger stature so lots of exercise for longer periods between kills
Thanks again Cort, for putting things in terms we can at least attempt to understand. Seems like this study fits nicely with the recent study you covered identifying the brain’s refusal to send work signals to our muscles (sorry, brain fog, but exercise preference, if I remember correctly). Our cells won’t respond so why would the brain ask it to do the work? The question is which came first, the response refusal or the brain refusal. I wonder if they’ve studied or found differences based on length or severity of disease like they have with the sex differences.
It certainly does seem to suggest a broken circuit somewhere in the brain doesn’t it? I’d be willing to bet the brain stem controls this process. It explains why it’s delayed. Once the body realizes it’s not getting the metabolites and proteins it needs to recover, it goes into panic mode.
Could that explain why there are CCIs being discovered in M.E people and treatment of that improving M.E symptoms
Maybe that’s why brain retraining works on some, but not all.
Maybe retraining only works on non pathogenic folks
I dont think the trigger matters. It seems like some circuit, or switch in the brain is stuck on. Maybe these people that can manually put themselves into parasympathetic and shift states can slowly turn that switch off. Cells need instructions on what to do. The brain gives them those, along with bioelectricity through ion channels. Emotions can creat that charge that gives the cells instructions. So, perhaps people have figured out a sort of way to back engineer the problem and manipulate it using energy and emotions. Michael Levin is at the forefront of this. He is quoted saying the future of medicine is gonna look a lot more like somatic therapy than chemistry. I found that fascinating.
i had an antibody test done to Herepesvirus 6 ab and the result was very high
1:2650 (antibody detected>1:10). I have had exercise intolerance 34 yrs. The lack of solutions in this time has led me to treating with herbs which help me manage and experimental alternatives but no solutions. I had an EBV viral panel with blood work at quest & think this was sent out but not super expensive.
Hi there. Which herbs help, may I ask? Would like to try!
i have been using an herbalist for years. my herbs are complicated because my system wants the exact right one which may be wrong the next day. further i take a mix of 24 herbs at a time and they are customized precisely for me so i couldn’t give you a list that would be helpful to you. i get herbs made up 2x’s a week.
I think ME/CFS is a very cruel disease. They just keep finding more and more wrong with our bodies.
It’s true- it’s quite extraordinary how many things are off – which makes sense given how more impaired people with ME/CFS are in general compared to other diseases.
The question is what is the tie that binds? What do all these things have in common? Energy disruption would probably be high on anyone’s list.
I have genital herpes EBV and cytomegalovirus and my fatigue episodes, usually triggered by exercise, are always prefigured by herpes tingles. I take a lot of valtrex which helps but does not cure.
Yes, looking at everything that is going wrong with us on such a very basic level, I find it pretty amazing that we are still even alive!
I agree with some others that there is likely some upstream ‘switch’ keeping us in this state, whether that’s to do with the sickness nucleus, the itaconate shunt etc. Research like this Hanson study is helpful and it’s all progress, but I think we would see more movement, quicker, if we could look at the upstream issues there are already theories about, instead of detailing every downstream problem possible before we follow them back up.
Also hoping they are looking at the high clusterin levels as being beneficially increased due to the increased damage in ME patients, not as something that needs reducing? Or at least considering that viewpoint? In the past I would have assumed they would, but some papers seem to avoid conclusions that seem obvious when you are living with the problem!
Thanks again for updating us all Cort 🙂
Thank you for your clear and careful presentation of this complex (for me anyway) information Cort. I really appreciate the audio version with the lovely narration too. 31 years on with this condition, knowing there are dedicated people like you and the scientific researchers still fervently working away to find answers fills me with hope, especially when findings like this emerge. I’m glad that the control group was sedentary – to hopefully counter the “de-conditioning” hypothesis! Of course there will still be the proposers of “traumatic childhood” “health anxiety” “type A personality” theories who swear they have brain-trained their way out of their own M.E./C.F.S. and that we would too if we could only believe/try/open our minds wide enough, but still…insights emerging from studies like these really lift my heart and fire me with hope. Thank you.
Hi Cort!
Thanks for another great write up – I’d never be able to take in all these studies on my own. You connect us all and bring us so much hope!
I’m really encouraged by the Hanson group’s findings and what they might find next – these studies are a bit like ending a brilliant TV series, as soon as you’ve consumed it you want the sequel!
Did the paper prospect about the exhaustion that results from other forms of exertion at all using its findings ie. cognitive exertion, energy used to digest a meal? For me, they actually flatten me way more than anything physical (I am severe and almost bedbound though so I’m not exactly active!)
I wonder if the exercise intolerance in ME has the same pathophysiology as other diseases that have exercise intolerance as a characteristic, such as metabolic and mitochondrial myopathies and – I think – some muscular dystrophies? Whenever I look at the symptoms of these diseases I’m struck by how much they overlap with ME. This website list a lot of them and it leaves me wondering if some of us could even have misdiagnoses:
https://www.mda.org/disease/metabolic-myopathies/signs-and-symptoms
https://www.mda.org/disease/mitochondrial-myopathies
I read that Byron Hyde thought many could be misdiagnosed – seems heartbreaking when there could be cures already but it’s so hard to get tested for all these rare and unusual diseases. There’s a study in the UK recruiting now that’s doing genetic testing for this reason:
https://bepartofresearch.nihr.ac.uk/trial-details/trial-detail?trialId=11083&location=&distance=
Do you know of a review paper covering the diseases that share features with ME at all Cort? I’d be interested to read it if it exists (or a blog on it even… :-)!) and see if some of the findings in those fields chime with, or expand on, what’s showing up in ME research.
One assumption I have harboured for a while is that the body “mothballs” cellular functions that it doesn’t have the fuel for, shutting down optional activities to preserve fuel for essential services. So seeing cells refusing to engage with exercise feels right.
So the symptom of apparent ‘fatigue’ is actually a protection from utter depletion by shutting down. Presumably there would be a biochemical pathway for this.
Your comment reminds me of Mark Davis’s suggestion that an exhausted immmune system may be draining energy from other parts of the body.
https://www.healthrising.org/blog/2024/05/18/chronic-fatigue-syndrome-long-covid-energy-sink/
Intensive exercise is bad for your immune system. Perhaps that is why herpes viruses can reactivate. Stress can also cause reactivation. Since ME patients are in overdrive mode, a little stress or physical exertion is too intensive.
Interestingly I was Found to be factor 13 deficient. My hematologist said it is so rare it’s hardly ever checked, but they were finding it in long covid patients. Would be curious if anyone else has had this test done, if not maybe it’s something to check. Her thought was it’s the body’s reaction to excessive clotting. When I had a tooth pulled I bled for 36 hours.
Also another clue why rapamycin could be helpful with the impaired autophagy.
Thank you so much for providing this information, and for including the “Gist” format for those of us with limited brain power for reading. This newsletter has become my one go-to source to keep me up to date on ME/CFS information. <3
Thanks, Christina 🙂
The Cornell team is on fire! Thank you Cort for an excellent review.
I wanted to point readers attention to Figure 5 of the study mentioning “LXR/RXR activation”, identified by Pathway enrichment analysis. LXR/RXR activation has been found to be relevant in COVID19 patients as well. Using machine learning and network analysis methods, LXR and apoptotic cell clearance have been identified as being potentially important for ME/CFS, as early as 2017 : https://algogenomics.blogspot.com/2017/09/more-genes-relevant-to-phagocytosis.html – COVID19 study : https://pubmed.ncbi.nlm.nih.gov/34511970/ |
“A failure to respond”…
More like….a failure to thrive!!!