Geoff’s Narrations
The GIST
The Blog
The GIST is in the middle of the page on the right

With studies popping up all over the place, plasmapheresis is getting a workout in ME/CFS and long COVID. (Plasmapheresis machine image – RexxS – from Wikimedia Commons).
The plasmapheresis possibilities continue! It’s amazing how many different blood cleansing options there are – and a shout out to Germany for leading the way in exploring them.
While the studies tend to be small and conflicting results are seen, at least we’re getting data on a possible treatment—something we’ve seen all too little of in ME/CFS, in particular.
Hold onto your seats – and be prepared for a bumpy ride – we’re digging into part II of the wild world of plasmapheresis in ME/CFS and long COVID.
The second and final (for now) part of Health Rising’s series on plasmapheresis possibilities for ME/CFS (chronic fatigue syndrome) and long COVID covers plasmapheresis aimed at removing antibodies (immunoadsorption), more on plasmapheresis aimed at cleaning up immune factors (TPE – with assist from Drs. Ruhoy and Kaufman), plasmapheresis aimed at removing blood clots and inflammatory factors – H.E.L.P. pheresis., and plasmapheresis aimed at removing specific autoantibodies (BC007)
Immunoadsorption
If autoantibodies are the ticket in ME/CFS and/or long COVID—and we don’t know that they are—then immunoadsorption (IA)—a form of apheresis focused on removing antibodies—could be the answer. Over the past couple of years, Dr. Scheibenbogen and her group at Charite University in Berlin have slowly been expanding their immunoadsorption trials.
Note that these are targeted trials: everyone in these studies has elevated B2ADR antibodies. Some studies suggest these antibodies could interfere with autonomic nervous system functioning, impact blood flows, and disrupt mitochondria.
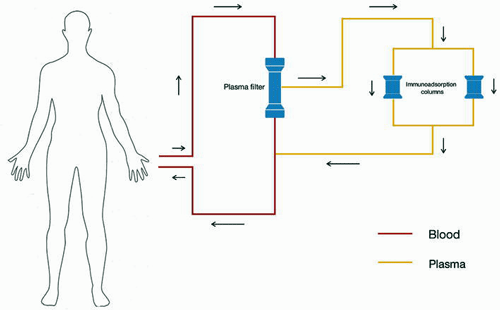
Immunoadsorption schematic (Image – Patrick Hamilton, Rhodri Harris, Mitra, CC-3.0, Wikimedia Commons)
An early proof of concept study found that immunoadsorption (IA): a) removed 80-90% of total IgG and the ß2AR-AB antibodies; and b) rapidly improved 70% of patients with three patients remaining improved 12 months later. A repeat trial two years later found that 4/5 patients again “showed a rapid improvement“. Immunoadsorption was off to a good start.
Next, the Charite group assessed 7/10 pretty severely ill (20-40 Bell scale) long-COVID patients with ME/CFS. Four patients reported a rapid and substantial improvement in their fatigue (SF-36: 30-45 points increase = a major enhancement in physical functioning) while 3 reported minor improvements, and one slowly built up to a major improvement. Two of the patients who reported rapid improvements showed a decline later on.
Prospective Long-COVID ME/CFS Study
In December 2024, the results of a prospective (non-placebo-controlled, non-randomized, etc.) trial involving 20 long-term COVID patients with elevated β2 adrenergic autoantibodies (β2 AR-AB) who met the criteria for ME/CFS (modified Canadian Consensus) were published.
The patients, who had an average disease duration of almost two years, received 5 immunoadsorption sessions over ten days at Charité – Universitätsmedizin in Berlin. Serum immunoglobulin levels and antibodies against β1, β2, M3, and M4 receptors were measured before, during, and after treatment. (Note that almost half the 400 long-COVID patients who met the CCC criteria for ME/CFS and were eligible for the study had elevated B2 AB levels).
Symptom assessments (SF-36, Bell Scale, DePaul Symptom Questionnaire (PEM), COMPASS31 (autonomic symptoms), handgrip strength and EndoPAT® measurements were used to evaluate muscle fatigue and vascular dysfunction. The 20 participants were followed up after six months.
Results

The plasma, a near colorless liquid in the blood, contains many different factors.
An almost 80% reduction in IgG and 77% reduction in β2 AR-AB indicated the immunoadsorption did its job in reducing antibodies. Seventy percent of patients were categorized as responders with an increase in the SF36 PF of at least ten points. A ten point increase in the SF-36 physical functioning domain is considered “clinically significant” and would likely “reflect noticeable improvements in daily functioning and overall physical well-being”. Still, a 10 point increase – which four of the responders achieved – is considered a “small effect”.
A closer look at the SF-36 PF results shows a familiar pattern. Six of the 20 participants had excellent results (30+ increase), while 4 had a 40+ increase—which is considered a dramatic improvement. One person shot up from 15-70, e.g., from being severely limited with a high dependency on others for daily tasks to having moderate limitations and being able to perform most daily tasks.
The authors also reported further lasting improvements in fatigue, post-exertional malaise, pain, cognitive, autonomic, and immunological symptoms. At month six, female patients had increased repeat handgrip strength. Seven patients who improved but relapsed in the following six months were retreated and responded similarly to their first round of IA.
While immunoadsorption did not return anyone to health, the fact that it was quite effective in some patients, moderately effective in others, and not at all in others highlights the need to target patients who could benefit from this expensive therapy.
To that end, the authors are doing comprehensive B cell subtyping, measuring more autoantibodies, and markers of immune activation, hypoperfusion, microclots, and SARS-CoV-2 persistence to see if they can pin down who benefits. One possibility the paper noted is that the responders had less severe muscular or mitochondrial impairment.
While treatments like immunoadsorption and plasmapheresis are almost always assessed by themselves, in the end, they will probably work best in conjunction with other treatments, and Dr. Ruhoy has some ideas about that (see below).
German Observational Immunoadsorption Long-COVID Study
In January 2025, an observational immunoadsorption study took place (non-placebo, etc.) of 12 long-COVID patients with ME/CFS symptoms and increased levels of 3 of 4 autonomic nervous system autoantibodies (β1, β2, M3, M4) and neurological impairments. Again, five sessions were done over ten days. This study, though, dug deeper into the effects of immunoadsorption on the immune system than ever before. T-cell stimulation, GPCR antibodies, viral titers, Epstein-Barr virus, cytokines, the spike protein, antibodies, and various symptom scores including SF-36 and COMPASS31 were taken.
THE GIST
- Hold onto your seats – and be prepared for a bumpy ride – we’re digging into part II of the wild world of plasmapheresis in ME/CFS and long COVID. The goal – to remove pathogenic factors found in the blood (er. the plasma in the blood) and return people with these diseases to health.
- The problem is that many quite different plasmapheresis techniques exist, none of which have been adequately studied yet. While inconsistent results abound, it seems clear that some people with these diseases can benefit quite a bit. The question is, which ones?
- Immunoadsorption (IA) removes antibodies, particularly adrenergic and muscarinic antibodies, in ME/CFS and long-term COVID patients. Early small studies were promising and suggested that five or so sessions of IA can produce long-term benefits in a subset of patients. IA ran into a roadblock recently when a new study found no symptomatic or biological benefits. However, a larger, more rigorous study is underway that is digging deep into the participants’ biology in an attempt to learn who benefits and how.
- Therapeutic Plasmapheresis (TPE)—In the last blog, we saw a small but rigorous study that found little benefit from TPE. On an Unraveled podcast (now on YouTube) last year, however, ME/CFS experts Dr. Ruhoy and Kaufman extolled the benefits of plasmapheresis. Dr. Ruhoy liked its broad spectrum of action and is using it in conjunction with IVIG to enhance IVIG’s benefits. Both hoped for more extensive studies. Dr. Ruhoy is offering it to her patients – and non-patients – in her Seattle office.
- BC007 aims to specifically remove autoantibodies to adrenergic receptors, and Berlin Cures touts it as a cheaper, more effective method of blood cleansing in these diseases. Anecdotal reports suggested the BC007 might be the real deal, but a government-funded, multicenter trial failed to meet its endpoints and was apparently stopped. Since then, however, another small BCoo7 trial has had good results.
- H.E.L.P. apheresis removes inflammatory proteins and blood clots (fibrinogen), allowing the blood to flow more fully in the larger blood vessels and capillaries. It also removes COVID-19 particles and both endo- and exotoxins.
- No ME/CFS studies have been done, but a 2023 German (of course) study involving 17 long-COVID patients showed promising results. While no validated symptom assessments were used, the authors reported that 16 patients experienced “significant improvement” and 12 nearly fully recovered.
- To Try or Not Try Some Form of Plasmapheresis – Applying the Arseneau Test. The “Arseneau Test” assesses a variety of factors to help decide whether or not to try a new treatment. It comes from a presentation given by Dr. Ric Arsenau, a Canadian ME/CFS/FM doctor. Since apheresis seems quite safe, the big question concerns whether the cost ($10-15K) justifies the potential benefits (ranging from none to quite significant) given the inconsistent results.
- If money is no object, I don’t see any reason not to try it. If I tried it, I would go with Dr. Ruhoy in Seattle, US, as she’s getting good results (and is surely combining it with other treatments). (Dr. Ruhoy’s fees have gone up dramatically recently, however.)
- People for whom money is an object have to weigh the cost, the potential benefits, and the inconsistent results.
So far, so good. Thirty days post-immunoadsorption therapy, though, while improvements in neuropsychological parameters and a small improvement in handgrip strength were seen, no improvements in symptom scores were seen (!). Plus, within a month, the autoantibodies were back.
About half the participants were believed to contain “active viral reservoirs” of the coronavirus. Despite the temporary removal of the soluble spike protein, no improvement in symptoms was seen. Indeed, the presence of the spike protein has not always been associated with symptoms in other studies.
From the Doctor’s Bench: Drs. Ruhoy and Kaufman on Plasmapheresis
In January and June 2024, Dr. Ruhoy and Kaufman of the Center for Complex Diseases in Mountain View, CA, and Seattle talked about their experience with plasmapheresis on their Unraveled Patreon podcast. (They have now moved to YouTube.) While they acknowledged that plasmapheresis is very expensive, it also has some interesting benefits. For one, it produces few side effects, does not suppress the immune system, and wipes stuff out of the bloodstream that triggers inflammation.

Dr. Ruhoy reported good results using plasmapheresis and likes to combine it with IVIG
By June 2024, Dr. Ruhoy had made plasmapheresis available in her clinic because, she said – it’s produced “such a great benefit for most of our patients” and because it’s so difficult to get it done anywhere else. She explained that besides not being approved for ME/CFS, plasmapheresis has been considered an “in-hospital” treatment and it can be difficult at times to get hospitals – even after it’s been prescribed – to do it in people with ME/CFS. (Dr. Kaufman said he’s found a doctor at Sutter hospital that will do it – and it gets covered…) Dr. Ruhoy was astonished.
Dr. Ruhoy said it’s much easier to do plasmapheresis now, and the machines are smaller and safer than when dialysis machines were required. Outpatient clinics are also popping up around the country.
Dr. Ruhoy has found that doing plasmapheresis before IVIG helps because IVIG provides “good antibodies” that aren’t blocked by the blood’s inflammatory substances (misfolded proteins, microclots, cytokines, toxins). Dr. Ruhoy loves the broad spectrum effects that IVIG and plasmapheresis have. She thinks that helping all pathways is probably more effective than going after one pathway, which is one reason why plasmapheresis has become such a big deal in the longevity world. Plus there’s the albumin factor. The albumin that replaces the plasma can be anti-inflammatory and improve immune functioning.
Dr. Ruhoy does six sessions over three weeks and will publish the results she sees in her office. You do not have to be a current patient (and in some cases, even a past patient) to do her plasmapheresis program. It requires a one-time intake appointment, which can be done via telemedicine.
Dr. Kaufman said if someone with a million dollars could support a study, he’d love to take 50 ME/CFS and long-COVID patients and monitor their outcomes with potential biomarkers. If successful, the study would be a step forward to getting insurance to cover it.
Courtney, in the comments to the podcast, wrote:
“This podcast was so interesting and as a patient on IVIG who has also received plasmapheresis, I am one more anecdotal example of how powerful this treatment is and positively impacts quality of life…Even if it is not a cure, it is adding years to my quality of life.”
Update: Since the podcast was done, one therapeutic plasmapheresis (TPE) study did not find any biological or symptomatic benefits. A 2022 French study that would have measured numerous parameters (muscle/blood vessel functioning, brain metabolism, cytokine activity, autoimmune markers) was withdrawn in August 2023.
How to reconcile these doctors’ enthusiasm for plasmapheresis with those study results? Perhaps Ruhoy and Kaufman have a different set of patients? Perhaps they’re better at picking who might benefit? Perhaps the other treatments they provide have a synergistic effect? Dr. Ruhoy noted that using plasmapheresis before IVIG improved the effects of IVIG.
Similarly, after stating that going after a pathogen while not supporting the immune system is often fruitless, Dr. Kaufman suggested we might be on the verge of a paradigm shift which includes using plasmapheresis and/or IVIG (or both) while going after the pathogen at the same time. Both doctors clearly support using multiple interventions.
The Unraveled session ended with the discussion of a formerly healthy and productive young man with long COVID (who has craniocervical instability) who is now bedbound, and the doctors wondering how many people realize the suffering that these diseases visit upon people.
BC 007
Interest in BC 007 rose from its ability to bind to and neutralize autoantibodies that attach to G-protein-coupled receptors (GPCRs). In their 2016 paper, Berlin Cures claimed their new approach was easier, considerably cheaper, and possibly more effective than past treatments such as immunoadsorption.

BC007 aims to wash out the autoantibodies blocking the activity of the beta adrenergic receptor (shown) (Image from David-Goodsell-CC_-3._Wikimedia_Commons)
Subsequently, several papers highlighted the drugs’ potential usefulness in “functional autoantibody diseases” such as complex regional pain syndrome, postural orthostatic tachycardia syndrome, ME/CFS, and others. The papers again asserted that other means of removing these autoantibodies such as plasma exchange, immunoadsorption (which they agree can be effective), intravenous IgG treatment (IVIG), or Rituximab were either more costly, less effective, or more toxic than using BC 007 aptamer. One report from the BR24 website said four patients with severe long COVID had been cured using BC 007.
Around 2022, the German government stepped in to fund a trial of BC 007 in 114 long-term COVID patients in centers throughout Europe. The researchers reported, ” We now have the opportunity to decisively advance our research in this important area.”
Late last year, though, it all seemed to come crashing down when a terse notice was made that the “topline results” of the phase II trial did not show efficacy over placebo, and the effort was stopped. Because the “topline results” consist of a high-level summary of the early finding of a clinical trial that focuses on the primary endpoints, we don’t know if BC 007 affected other symptoms. Topline results are typically used to determine whether to proceed with the trial. It’s a shame that we don’t have more information.
The reCOVer BC 007 Trial
Given the ups and downs we’ve seen with plasmapheresis, maybe it made sense that we’d see a positive result next! Sarah Frischke’s German “Living with ME/CFS” website noted that another, smaller BC 007 clinical trial—the reCOVer study at the University Hospital Erlangen, led by Dr. Bettina Hohberger—was underway. The results of this randomized, placebo-controlled, double-blinded, crossover BC 007 trial were published in a preprint published in December 2024. Thirty patients received one infusion of BC 007.
Various symptom questionnaires (Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue Scale and Chalder Fatigue Scale (for fatigue), the Bell scale and Fatigue Severity Scale (FSS, for severity of fatigue), the Canadian Criteria (CCC, for the presence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)), SF-36 questionnaire (for quality of life), and 6MWT (Borg scales), the 5-item DePaul Symptom Questionnaire – Post-Exertional Malaise short form 8), and a six minute walk test were done.
Because it was a crossover study, it was difficult to determine how much improvement was made, but the authors stated that the FACIT fatigue scale, Bell scale, and FSS questionnaires indicated that significant improvement in fatigue was seen. (The Chalder fatigue scale, on the other hand, did not show any improvement.)
They also noted—as other studies have—that determining which patients will benefit will be crucial. It didn’t seem likely that anyone would be cured, but the authors concluded that “BC007 might offer a novel curative and causal therapy for an autoimmune subgroup of patients with PCS.”
The authors proposed that the different study designs, fatigue scales and durations of illness may have explained the different results in these trials.
H.E.L.P. Apheresis
H.E.L.P. Apheresis (Heparin-induced Extracorporeal Lipoprotein Precipitation) uses a centrifuge, heparin and an acetate buffer to reduce plasma pH and precipitate lipoproteins (proteins that bind to fats) such as LDL cholesterol, lipoprotein(a), and VLDL, along with fibrinogen, out of the plasma. The goal with long COVID is to increase cardiovascular health by removing inflammatory proteins and blood clots (fibrinogen) thus allowing the blood to flow more fully in the larger blood vessels and capillaries as well as removing COVID-19 particles and both endo- and exotoxins.
No ME/CFS studies have been done, but a 2023 German (of course) study involving 17 long-COVID patients showed promising results. The study provided as many H.E.L.P. treatments (up to 7) as necessary to improve health. No validated symptom assessments that I could tell were used. The authors reported that 16 patients experienced “significant improvement” and 12 nearly reached full recovery.
Patrick Usher wrote a blog post on Health Rising about his experience of 7 H.E.L.P. sessions. He believes he improved by about 20%.
To Try or Not Try Some Form of Plasmapheresis – Applying the Arseneau Test
The “Arseneau Test” assesses a variety of factors to help decide whether or not to try a new treatment. It comes from a presentation given by Dr. Ric Arsenau, a Canadian ME/CFS/FM doctor.
- The credibility of the source – several published papers and two respected doctors – the credibility is fine.
- The quality of the evidence – mixed – the inconsistent results of the small, sometimes methodologically strained studies (par for the course for these diseases) makes it difficult to know what to think.
- The benefit / cost analysis – the benefit ranges from none to mild to significant with few expectations of a full return to health. The expense is high. From what I could tell, six courses of plasmapheresis cost roughly $15K in the US, a 5-course of INUSpheresis or H.E.L.P. apheresis in Germany / Austria costs roughly the same, a H.E.L.P. course in the US costs about 10K, and an immunoadsorption course in Germany costs @ $10K US dollars. BC 007 is reportedly much cheaper but does not appear to be commercially available.
- The risk-benefit analysis – low risk and low to moderate to high benefits. The treatments appear to be well tolerated; the major question is their effectiveness.
Conclusion — Blood cleansing offers little risk and a significant potential upside—if you can afford it. However, its high costs and the lack of clarity regarding who benefits mean trying it comes with a substantial financial risk. The fact that it may need to be repeated should be considered.
If my financial coffers were flowing over, I’d probably give it a try, but in the U.S. I would do so while being treated by Dr. Ruhoy and/or Dr. Kaufman, given their positive experiences and the other treatments they’re employing. The fact that you don’t need to be a patient to get plasmapheresis at Dr. Ruhoy’s Seattle office may help those unable to afford Dr. Ruhoy’s new fees (5K/hour). (I have been told, though, that you can go from month to month on her new concierge program, which might help.)
While that’s a lot of money, it doesn’t take much listening to hers and Dr. Kaufman’s Unraveled Patreon/YouTube sessions to get that these doctors are on the cutting edge of ME/CFS and long-COVID treatments.
Conclusion

Plasmapheresis is an expensive option but does seem to help some patients. The question is which ones. An immunoadsorption study should help.
The good news is that blood cleansing trials – almost all hailing from Germany – are being done in ME/CFS and long COVID. The bad news is that most are small, some methodologically suspect, and the results have been inconsistent.
While we can’t draw any big-picture conclusions regarding blood cleansing’s efficacy in ME/CFS and long COVID, it does seem possible to draw a “little-picture” conclusion; i.e., that in the right person, blood cleansing can be quite helpful.
Unfortunately, the biggest study done so far—the multicenter BC 007 study—is something of a mystery. With regard to the others, we’re furthest along with immunoadsorption, which has had several small trials, and an impressive larger study is underway.
The 66-person, double-blinded, randomized, sham-controlled, exploratory immunoadsorption trial taking place at Charite University in Berlin is assessing a wide range of parameters(routine blood and urine tests, viral serology, pregnancy, toxicology screening), cerebrospinal fluid, and blood samples—autoantibodies, markers of neurodegeneration, and neuroinflammation.
Physical assessments (finger tapping, handgrip strength evaluation, 6 Minute Walking Test (6MWT)), autonomic assessments (Schellong test), and numerous questionnaires and tests (Chalder Fatigue Scale, the Patient-Reported Outcomes Measurement Information System (PROMIS), Post-COVID Functional Status (PCFS) Scale, The Montreal Cognitive Assessment (MoCA), and Symbol Digit Modalities Test (SDMT) are also being done.
Plus, research is underway in other trials to pinpoint who benefits and who doesn’t. So, while the blood cleansing field is chaotic right now, hopefully we’ll be on sounder ground soon.
- Coming up – someone with an autoimmune disease finds a much cheaper way to improve her immune functioning and health.





Again, not buying into this!
🙂 Got it!
An Australian doctor named Dennis Lau is also running a plasmapheresis trial; in POTS.
https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=387690
Thanks for posting the link. I also live in The Land Down Under 🙂
This should be interesting to see if it produces positive results for POTS. Also, that they will be doing a comparative treatment with intravenous fluids (Hartmann’s solution} along with it.
I have had POTS (along with ME/CFS for 32 years), and I find that this is the most disabling condition I have to deal with! It is very debilitating – in not being able to remain upright for more than a few minutes.
The INUSpheresis gurus recommended 2 treatments with a day or two off in-between them, for which the clinic in Frankfurt charged 5200 EUR. As I told them at the time, the treatment would have to make me well long enough for me to start working again for me to afford repeated treatments.
Cort, are you aware that in the U.S. some Functional doctors are using a machine that will do ultraviolet blood irradiation (UVBI)? I read about this and found a clinic that uses this method, in addition to EBOO and Photodynamic Therapy to treat Fibromyalgia, Chronic Fatigue, Long Covid, etc. I have benefited greatly from these treatments. Currently, my fibro pain is completely gone and we are now focusing on my fatigue. There are no drugs involved and no side effects.
In my search for a clinic I found there are not very many that use these methods. Also, these treatments are not a one time thing. Most people need several treatments.
I t’s great to be able to benefit from these methods, because at the base, UV blood irradiation treats blood cancer and graft disease, photodynamic therapy treats skin cancer.
UVBI treats sick cells.
Are our cells sick too? deficient?, old? which prevent the new (young) healthy cells from renewing themselves?
Please keep us informed Louise, from the rest of your treatment. There is a clinic near me which has the UV blood irradiation apparatus. I have award to the neuromuscular service for rare disease in the same place. I will touch a wordcancer de la peau.
L’UVBI traite les cellules malades.
Bad translation; At the end of the text, I meant; ‘I’m going to find out about the UV device for EM/SFC’, not for skin cancer. Sorry
When you say chronic fatigue do you mean the symptom of many conditions?
I have Myalgic encephalomyelyetis/Chronic Fatigue Syndrome and the concomitant PEM. I know the terminology varies and is a pain, but chronic fatigue is a bug bear of mine. Sorry.
Jane, I have Chronic Fatigue Syndrome.
Yes I thought that’s what you meant but it’s misused so often xx
I’m in the US and I did about 6-8 sessions (lost count) of ultraviolet blood irradiation (UVBI) with added ozone. Functional Medicine Doctor indicated this would treat reactivated EBV. After a whole lot of money spent, it did absolutely nothing to change EBV lab values or ME/CFS symptoms.
See. https://www.amazon.com/Understanding-CFS-Strategies-Healing-Breakthrough-ebook/dp/B0DM1194R5?dplnkId=836c9de1-7d46-413b-8d9a-172f0042a104
New book out on CDs and the German research
I am reluctant to do plasmapheris treatment with Dr Tuhoy even though it sounds positive. I’d rather make sure I did it with the ivig and see some published results. She’s certainly charging a lot of money a red flag for me. I am going to try EBOO instead locally…cheaper. has anyone else benefited from EBOO?
Ruhoy sorry typo
I did 8 Ozonotherapy sessions in IV. It didn’t do anything for me, just getting tired even more because of the trip. Maybe it takes many more sessions?
https://www.goodreads.com/book/show/225055290-understanding-me-cfs-strategies-for-healing
Thanks! Looking forward to it. 🙂
Thanks Cort.
Two aspects I recommend testing before plasmapheresis:
Investigating the cause of excessive red blood cell count (high RBC) in many cases.
Assessing for the presence of vascular disease, specifically Antiphospholipid Syndrome (APS), using the ELISA test—especially since heparin is involved.
One more INUS Medical Center in Germany
Individually adapted and sustainable therapy concepts
https://www.inusmedical.de/start-englisch/
Yes, cytokines play a key role, but there is no definitive conclusion on whether the type I interferon (IFN-I) system is dysregulated in various autoimmune rheumatic diseases. These include systemic lupus erythematosus (SLE), primary Sjögren’s syndrome (pSS), rheumatoid arthritis (RA), systemic sclerosis (SSc), and dermatomyositis (DM).
Please consider reading:
Prevalence of EBV, HHV6, HCMV, HAdV, SARS-CoV-2, and Autoantibodies to Type I Interferon in Sputum from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients https://www.preprints.org/manuscript/202502.0185/v3
Best Sieglinde
Thank you, Sieglinde, for offering the link to this new latent viruses study from Sweden. On the one hand I am satisfied that we’re seeing more and more herpes and other latent viruses studies in ME/CFS. I wonder though whether they are desigend well enough to actually bring forward the results we want to see.
My critique is that most study designs to not differentiate acute and non-acute states in chronificied ME/CFS when on the other hand their participants are long time ME/CFS patients (in the study anything between 8-28 years since onset).
Why is that a problem? I wonder if any of these patients had an acute ME/CFS inflammatory episode during the period their testing samples were taken.
Myself, I am seven years in and I haven’t had more than three flare-ups of not even more than a couple of days over the past two years because I rest and pace so well in the meantime.
I am very happy that Jackie Cliff from Brunel University and her team who are currently looking into HHV-6b understand well that ME/CFS is a chronically recurrent illness where every severe episode throws you deeper into the illness or at least way back in your recovery. When at the same time the better you pace the less acute phases you experience.
I think that EBV might not be our answer, and that Cliff is right that the severe acute phases are driven by HHV-6b.
I hope that the important fact of acute and non-acute phases is going to be discussed and better reckognized in May at the big ME/CFS conference in Germany.
Hi Lina,
You raise the same question I’ve been asking for years. In my case, I don’t have EBV, but ELISA confirmed HHV-6, and I experience all the classic ME/CFS symptoms. The narrow focus on EBV has marginalized other potential research avenues, ultimately stifling progress in understanding symptom-related exhaustion.
For example, I’ve had surgery-related sepsis four times in my life, and each time, my muscle weakness worsened. Ineffective treatments, like graded exercise therapy, only exacerbated my skeletal and leg muscle deterioration. However, extended rest and cytokine-reducing medications allowed me to regain partial function—until another virus reawakened my already compromised immune system.
Many ME/CFS patients cling to the idea that EBV is the root cause, yet many have never been tested for it—or for other viruses and bacteria that could impact the immune system and alter gene expression. Clinical medicine often falls short due to time constraints, financial limitations, or a lack of education on how epigenetics could guide diagnosis and treatment.
Through my research, I’ve come across many related illnesses that could explain why pacing helps reduce muscle weakness. In my case, it turned out to be dysferlinopathy, a progressive deterioration of the sarcolemma. The key to answers may lie in epigenetics—especially since I have the DYSF gene. This could be confirmed through a biopsy, but insurance would not cover it.
If you would like to read more about:
https://swaresearch.blogspot.com/2025/01/dysferlin-protein-key-roles-locations.html
Ooops, I am actually eight years in in April with four years of a mild course and then a fast deterioration since the end of 2020. Officially diagnosed only two years ago.
I have been following the research of ptofessor Scheibenbogen et al. with interest for a long time. Her findings are more objective than many other studies, which makes them interesting. It should be noted that they mainly concern POTS/ME patients. The group with increased antibodies, particularly against adrenal receptors. Some time ago I spoke to an immunologist about her findings. If I understood correctly, this does not mean that there is an autoimmune disease. He did note that there is something immunologically wrong.
The results of plasmapheresis remind me of the studies of stem cell transplants….very expensive procedures that seem to help some patients, but temporarily. This suggests that something in the body is continually corrupting many functions and that as soon as the treatment wears off, it is back to square one.
I think this is why Dr.Cheney developed MTF cell factors. This powerful stem cell treatment kicks the body back into better function, but it can exhaust you for a few days after even a tiny amount of the gel on your foot.
This treatment is made from pig liver as is the Nexavir that I take every day. I actually have both treatments and add the MTF if I have overdone it.
Both of these treatments are inexpensive compared to stem cell transplants and/or plasmapheresis.
Nexavir is available without a prescription from the Nexco Pharmaceutical company and the stem cell factors can be purchased from Dr. Cheney’s family that holds the patent.
Raelan agle now has over 200 CURED cases with brain retraining.
Somethings not adding up
The fact that this ruhoy charges 5k per hour is a HUGE red flag.
In the 90s I was sent to a large Canadian city by a charity. The treating physician gave me iv vitamins that I didn’t respond to.
The physician charged the charity 20k for 8 iv vitamin treatments that took one hour each to administer. I found out later from a nurse that one bag of iv vitamins amounted to 12$
That’s why, from a monetary perspective alone, I think it makes sense. even if the success rate is low, to try brain retraining. An upcoming blog will show how a person with an autoimmune disease who did not respond to drugs dramatically improved her health using a variety of mindfulness practices.
Cort, your the best!
Nevermind a may 12th me/cfs day…
We need a Cort day of recognition
Hi Cort,
Can you tell me how to reach the Sutter system treatment, which you say is
“covered” I assume by insurance)?
What is the Sutter system treatment? I don’t recall (???)
While I support capitalism, $5k an hour seems like price gouging a vulnerable population looking for any possible hope available.
I imagine that we’ll learn shortly why the huge price increase. I had just started seeing Dr Ruhoy and she quickly identified a longstanding problem that no one else had identified (hiatal hernia (oddly enough it was one of the first ideas I had when I first got ill decades ago – but it took Dr. Ruhoy to diagnose it.).
It was wrenching to get the email which basically indicated that neither I or my partner will be seeing her long term.
She ordered a bunch of tests I had never heard of and is trying out new things and is very up on the subject. It’s a pity it’s going to stop for us. I’m sure we are not alone.
$5000 an hour. Are you kidding me? And patients used to complain about Dr. Cheney’s costs. At least his treatments worked, at least, for me. Has Dr. Ruhoy actually cured any ME/CFS or Long Covid patients?
Mindfulness, give me a break. I was coping pretty well with ME/CFS until two bouts of Covid in the same year affected my heart, blood pressure and hearing as well as my balance and ability to walk. I don’t thinking “happy” thoughts is going to help.
Even the most elite, greediest lawyers who bill billion dollar corporations don’t charge rates that high.
Muscarinic receptors are part of the parasympathetic nervous system, which controls functions like digestion, heart rate, and bladder function.
They respond to acetylcholine.
They are also involved in various physiological processes, including smooth muscle contraction, glandular secretions, and heart rate regulation.
Autoantibodies against muscarinic receptors, particularly M2 muscarinic receptors, have been implicated in conditions like chronic fatigue syndrome and autoimmune orthostatic hypotension.
The presence of these autoantibodies can be associated with various autoimmune and inflammatory conditions.
Research also suggests that these autoantibodies may play a role in the development and progression of diseases like myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS).
The association of autoantibodies with immune markers suggests that they activate B and T cells expressing β adrenergic and M acetylcholine receptors. Dysregulation of acetylcholine and adrenergic signaling could also explain various clinical symptoms of CFS.
Disruptions in acetylcholine (ACh) signaling can stem from various factors, including toxins like nerve gases and pesticides, certain medications, diseases like Alzheimer’s and Myasthenia Gravis, and even the venom of certain spiders.
This may be why pyridostigmine helps in some cases like GW Syndrome where the veterans were exposed to pesticides and many other toxic chemicals.
Pyridostigmine is an acetylcholinesterase inhibitor that slows the breakdown of the neurotransmitter acetylcholine in the synaptic cleft. This increases the amount of acetylcholine.
My eyes got opened very wide when I watched dr.murray vimy you tube videos on how mercury amalgams release toxic gases of mercury directly into every organ in the body.
He placed mercury amalgams into the mouths of sheep knowing sheep chew very similar to humans.
He knew the liars trying to cover up this fraud would come back saying sheep are not even close to humans so he also did the same installation of mercury amalgams into monkeys teeth
The amount of toxic mercury being released skyrocketed the more the monkey chewed food. The mercury again ended up in every organ of the monkey.
If you are a tooth grinder while sleeping the amount of mercury gases being released increases dramatically.And, if your as old as me,we would have gotten another dose of mercury in our childhood vaccines.
And,let’s not forget, while they have removed lead from auto gasoline, they have not removed lead from aviation fuel
Hal Huggins and other integrative dental researchers did much of this kind of research about forty years ago; it was considered quackery until relatively recently. Some people do hair heavy metal testing through practitioners like Dr. Amy Yasko, and also Amy Myers/Detox site; some use MCP/Modfied Citrus Pectin to pull out all inflammatory toxins from the body while it leaves the good nutrients in. A therapeutic dose needs to be used until a few tests show that more metals are not accumulating.
One other approach to detoxing is injectable ozone therapy at a holistic dental office with a doctor with a solid track record of this helping to eliminate infected bone pockets left over from improperly done root canal surgery. Even old wisdom tooth extractions are suspect. Hidden infections in jaw bone can be another cause of mysterious longterm fatigue that does not respond well to conventional or holistic medicine. These cavitation situations may be hard to diagnose, but they are another bullet point on the laundry list when solutions are hard to find. In TCM, each tooth is connected to an acupuncture meridian, and an inflamed hidden cavitation can disrupt an entire organ system, for those who understand and apply the principles and practice of Chinese medicine.
Here in the German speaking countries the different procedures of blood wash were covered tightly by the media in early cases of long COVID. When I look back to these TV documentations it seems to me that the patients who told the journalists that they had benefitted greatly from the procedures would probably have recovered even without a blood wash.
They were typically wealthy, healthy, and socially well positioned people often themselves from the medical field. They also were informed about rest and pace as a cautionary measurement early on and had the means to do so.
I am not surprised that we had these probably genuine stories of these people who thought they had benefitted from the blood washes and at the same time we’re now finding out that it doesn’t work for the chronificied state of ME/CFS.