

Geoff’s Narrations
The GIST
The Blog
We’re back to the B-cells, and that brings us back to the Charité 3rd International ME/CFS and long COVID Conference. B and T-cells are the two main players in the adaptive or late immune response. They’re the cells we depend on to wipe out infections, and when they go bad, really bad things can happen.
Key players in the adaptive immune response – when B-cells go bad, they can really go bad.
ME/CFS studies have found stressed B-cells, which lack the energy to function normally; immature B-cells that just don’t want to grow up, or go on the attack (high CD24 levels); proliferating B-cells with reduced mitochondrial mass; which appear to be chronically activated by infections; as well as overactive B-cells.
B-cell study is in its infancy in fibromyalgia. Still, Andreas Goebel’s recent preprint suggests fibromyalgia exhibits problems in B-cell tolerance (exaggerated B-cell proliferative responses, enhanced clonal expansion, autoantibody production) that mirror those seen in autoimmune diseases. Plus, a recent gene expression study found highly activated B-cells in FM that were pumping out interferons and inflammatory cytokines at a high rate.
In long COVID, increased levels of activated B-cells, increased plasmablasts (antibody-producing B-cells), and functionally impaired B-cells suggest that B-cells have been chronically turned on (and are suffering for it).
Note that in all these studies, evidence of chronic B-cell activation has emerged. That’s not a great finding given what the IgG transfer studies have found.

New drug – new possibility
The GIST
- The Rituximab study didn’t go so well, but Fluge and Mella haven’t given up yet. At the recent Charite International conference, Fluge presented data on a new B-cell depleting drug called daratumumab.
- Studies suggest that chronically activated B-cells are causing problems in ME/CFS, FM, and long COVID, Indeed, when things go wrong with these major players in the immune response, bad things can happen. B-cell problems play a role in a wide swath of major diseases, including lupus, MS, and rheumatoid arthritis.
- Activated B-cells produce immunoglobulins (IgGs, also known as antibodies), and IgG/antibody transfer studies in ME/CFS, fibromyalgia, and long COVID consistently suggest that something pathogenic has gone awry with their IgG production.
- These findings have prompted attempts to filter out or remove bad B-cells in various ways (Rituximab, cyclophosphamide, immunoadsorption/plasmapheresis, IVIG, daratumumab). The Open Medicine Foundation’s TREATME survey found that IVIG was the most efficacious treatment.
- Convinced that a different B-cell depleter would work better, Fluge and Mella produced a small 10-person pilot trial of daratumumab (Darzalex), which targets a different B-cell than Rituximab, that followed patients for at least a year.
- The results were stark: about half the patients improved dramatically, many reaching a normal level of function, while the other half didn’t improve at all. The improvements began 6-8 weeks after the first injection (no placebo was used here!) and continued to increase over time.
- An analysis suggested that dramatic drops in IgG4 antibodies may have been responsible. High IgG4 levels can lead to an overproduction of collagen, resulting in the excessive growth of connective tissue. That’s an interesting finding given the collagen thickening in the capillaries found in the Wust group’s recent muscle study, which could be impairing blood flow. Indeed, high IgG4 levels can negatively affect the blood vessels in numerous ways, including impairing blood flows.
- While high IgG4 levels were not found at baseline in these patients, high antibody levels aren’t always necessary to cause disease.
- Given the small size of the study and the absence of placebo controls, the authors noted, “No definite conclusions should be drawn before a randomized study has been performed.” but have applied for a 66-patient, randomized, double-blind, and placebo-controlled ME/CFS study (including long-COVID patients who meet ME/CFS criteria (CCC)). They hope it will be approved shortly.
- Another B-cell depleting study – on Rituximab, of all things – is underway in Japan. Despite the fact that Rituximab failed in ME/CFS, the authors of that study wrote that “our pooled experience supports that some ME/CFS patients seem to respond to rituximab and B-cell depletion“, and noted that the maintenance dose in the large study had been reduced.
- The Japanese study will utilize standard maintenance doses and conduct a range of assessments, including brain, microbiome, immune, and metabolic evaluations, to determine who benefits.
Health Rising’s Quickie Summer Donation Drive is on the Brink!
Thanks to everyone who has supported Health Rising thus far. In the last blog of the drive, we are on the brink of meeting our target. 🙂
Activated B-cells make immunoglobulins (IgGs, aka antibodies); and IgG/antibody transfer studies in ME/CFS, fibromyalgia, and long COVID consistently suggest that something pathogenic has gone wrong with their IgG.
IgG from people with ME/CFS has fragmented mitochondrial and damaged endothelial cells in culture. Mice given fibromyalgia IgG exhibit increased pain response, reduced grip strength, and small nerve fiber damage. Long-COVID IgG has been shown to produce reduced activity, increased pain, dizziness, and sensory stimulation in mice.
These findings have prompted attempts to filter out or remove bad B-cells or their antibodies in various ways (Rituximab, cyclophosphamide, immunoadsorption/plasmapheresis, IVIG, daratumumab). The Open Medicine Foundation’s TREATME survey found that IVIG was the most efficacious treatment.
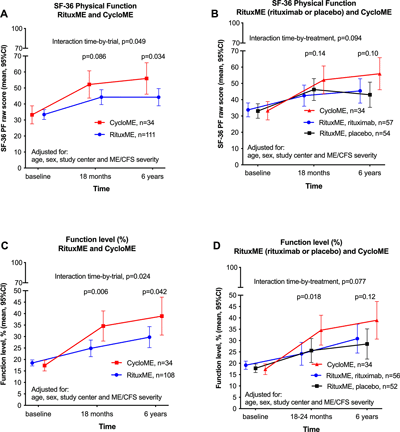
A different B-cell depleter, cyclophosphamide (red), proves more effective than Rituximab (blue) in ME/CFS.
The Cyclophosphamide Lesson
Probably because it’s too toxic for widespread use, Fluge and Mella’s successful trial of a B-cell inhibiting drug called cyclophosphamide hasn’t gained much attention.
A mustard compound that emerged during research on chemical warfare, cyclophosphamide was originally developed to treat cancer, but it’s also a highly effective B-cell depleter, which depletes different kinds of B-cells than Rituximab.
Note how much better cyclophosphamide performed in ME/CFS than Rituximab did.
Given the huge role B-cell dysregulation plays in many diseases (lupus, multiple sclerosis, Sjogren’s Syndrome, rheumatoid arthritis, type I diabetes, Crohn’s disease, chronic hepatitis, etc.), it wouldn’t be surprising at all if something was off with the B-cells in the ME/CFS/FM/long-COVID suite of diseases.
The mAbs (Monoclonal Antibodies)
It was Fluge and Mella who got the ME/CFS Rutuximab studies going in Norway and it’s back to Norway we go with a new study using a different mAb called daratumumab. Fluge, Mella and Tronstadt now believe they know why the big Rituximab ME/CFS trial failed: it targeted the wrong B cells.
The autoantibody picture is somewhat mixed, but adding IgGs from patients with ME/CFS, fibromyalgia, and long COVID to mice and culture suggests something pathogenic is present.
Rituximab targets CD20 B-cells, which tend to have a shorter lifespan. The authors believe, however, that the autoantibody production found in ME/CFS is likely coming from longer-lived B cells, which daratumumab targets.
They believe that alterations in the levels of “functional autoantibodies”, i.e., autoantibodies that play a positive role in the body, as opposed to “reactive autoantibodies”, autoantibodies that attack the tissues, are in play in ME/CFS and long COVID.
The autoantibody evidence is mixed. The functional autoantibodies believed in play in ME/CFS – those that impact the beta-adrenergic receptors (β1, β2 AdR) and/or muscarinic cholinergic receptors (M3, M4) – are complex and difficult to measure. Some studies suggest they are contributing to the dysautonomia in a subset of people with ME/CFS. Other studies have not found that. Using a sophisticated testing regimen, Hanson’s group recently did not find evidence of upregulated autoantibodies.
It’s possible, though, that autoantibody balances may be more important than single autoantibody levels.
The Daratumumab (Darzalex) ME/CFS Study
https://events.mecfs-research.org/en/events/conference_2025/videos/oystein-fluge-plasma-cell-targeting-daratumumab-mecfs
The goal of the “Plasma cell targeting with the anti-CD38 antibody daratumumab in myalgic encephalomyelitis/chronic fatigue syndrome—a clinical pilot study” study was to wipe out autoantibody-secreting B-cells in an attempt to improve blood flow, reduce preload and hypoxia, and improve symptoms.
Because B-cells they were after can live for decades in the bone marrow or gut wall, the authors hoped that altering levels could produce long-term changes.
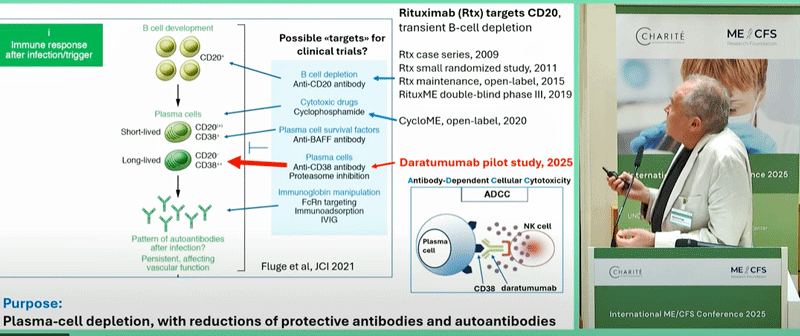
The Norwegian B-cell depletion saga from Oystein Fluge.
The Study
The small study followed 10 female ME/CFS patients who received daratumumab injections (1,800 mg) at weeks 0, 2, 4, and 6, with added maintenance treatments at weeks 14, 22, and 30 for the last four patients.
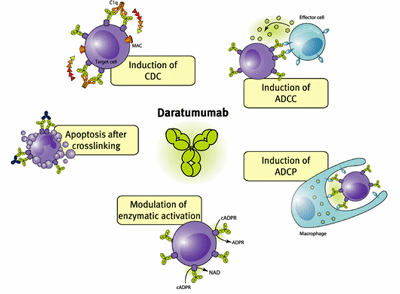
Image by Niels-W.-C.-J.-van-de-Donk,-Maarten-L
Prior to the first injection, dexamethasone 10 mg orally, cetirizine 10 mg orally, paracetamol 1 g orally, and montelukast 10 mg orally were given. Two days after the injections, they received dexamethasone 4 mg orally. If patients experienced no adverse reactions to the first daratumumab injection, dexamethasone 10 mg, cetirizine 10 mg, and paracetamol 1 g were administered prior to the next injection.
They were followed for at least 12 months, and in some cases for 24 months.
Results
The results were impressive. Six out of ten patients experienced “marked improvement”: i.e. SF physical function score more than doubled (!). The improvement from 32 to 78 translates to going from having major difficulties with moderate activities or self-care tasks to a level of functioning that’s close to normal (!). It got even better. By the end of the study, five of the six improvers ended up with a mean SF-36 PF of 88 (range 80–95) – indicating they had “almost no physical limitations”. Five of the responders were able to maintain a mean step count above 10,000/24 h for some weeks at a time.
The improvements began 6-8 weeks after the first injection (no placebo here!) and increased over time.
The patients who didn’t improve showed no changes at all. There was no guessing who did or did not improve; either a patient did really well or did not improve at all.
Surprise Impact
Biologically, the study aimed to reduce serum IgG levels. Patients who improved had a more pronounced reduction in IgG antibodies (54%) than those who did not (40%). Most interestingly, it was the smallest subclass of IgG antibodies, IgG4, which decreased the most in those who improved. IgG4 antibodies were reduced by 65% in responders but only by 29% in non-responders.
These seemed a bit odd as IgG4 is also the only IgG antibody class that has not been linked to the beta-adrenergic (β1, β2) and muscarinic (M3, M4) autoantibodies these researchers were aiming at. Only one ME/CFS/long-COVID study has highlighted high IgG4 levels.
Yet, this preliminary study suggested that daratumumab may have achieved its good work by knocking down IgG4 antibodies.
I’m particularly interested in IgG4 because I have consistently very high IgG4 levels, which can drive tissue inflammation and fibrosis. In IgG4-related disease (IgG4-RD), the tissue inflammation often also drives mast cell production, which then interacts with IgG4 to produce more inflammation.
Indeed, high IgG4 levels can cause an overproduction of collagen and growth of the extracellular matrix, resulting in the excessive growth of the connective tissue. That’s an interesting finding given the collagen thickening in the capillaries found in the Wust group’s recent muscle study, which could be impairing blood flow. High IgG4 levels can negatively affect the blood vessels in numerous ways including impairing blood flows.
Of course, IgG4 antibody levels do not need to be high to drive disease. Even low levels of highly specific autoantibodies can produce major effects (myasthenia gravis, Graves disease) in some diseases. Antibodies can also have localized effects that are not reflected in serum samples. Note that high IgG4 levels were not found at baseline in these patients, but reducing IgG4 may have played a key role in the patients’ recoveries.
Higher NK cell numbers at baseline were associated with a better response.
While daratumumab is usually used in cancer, the authors noted a series of case studies suggesting it can be highly effective in autoimmune patients who have not responded to treatment. Because those studies suggest that the first daratumumab injection may be the most important one, they’re beginning a new series of tests using fewer doses in four long-COVID ME/CFS patients (with higher baseline NK cell levels).
Given the small size of the study and the absence of placebo controls, the authors noted, “No definite conclusions should be drawn before a randomized study has been performed.” but have applied for a 66-patient, randomized, double-blind, and placebo-controlled ME/CFS study (including long-COVID patients who meet ME/CFS criteria (CCC)). They hope it will be approved shortly.
New Rituximab Study Underway
A new 30-person Rituximab study is underway in Japan. One might ask, “Why?” Fluge and Mella’s six-year update of the 2017 151-person double-blinded Norwegian Rituximab study found no evidence that Rituximab had positive effects.
Yet Fluge and Mella’s followup study also stated that “our pooled experience supports that some ME/CFS patients seem to respond to rituximab and B-cell depletion“, and provides some reasons why the big Rituximab study may have gone bust.
Patient heterogeneity is probably always going to be high on anyone’s list as we don’t know how to biologically target patients for drug trials, but the authors also noted the lower maintenance doses of rituximab used than in the previous phase II studies. (I read somewhere that funding was an issue in that.)
The Japanese speaker did not explain why they chose to do another Rituximab study, but the new study will adhere to traditional maintenance doses and focus on exploring Rituximab’s effect on the gut microbiome, the brain (both SPECT and MRI), the immune system (flow cytometry, B-cell repertoire, a couple of autoantibodies), and metabolomics. If it is successful in at least some patients, it may help tell us which patients are more likely to benefit.
- As a side note, a case report just appeared on a person with a diagnosis of ME/CFS and later multiple sclerosis who responded well to Rituximab after other treatments had failed.
The Past is Not Necessarily Prologue

New drug – new possibility.
Some people will probably look at Fluge and Mella’s two successful small Rituximab studies and then the big Rituximab study that bombed and throw up their hands and assume the same pattern will occur with daratumumab.
Time will tell what happens with daratumumab, but consider that it affects B-cells differently, that Fluge/Mella and the Norway team picked it because they feel they know more about the B-cell situation in ME/CFS now, that another B-cell depleting drug (cyclophosphamide) appears to be helpful, and finally, that this is how science proceeds.
It’s a pity that it proceeds so slowly for ME/CFS, but perhaps it’s not surprising that the first try at a B-cell drug didn’t go so well.
Conclusion
Studies including IgG (antibody) transfer experiments suggest problems with the B-cells exist in ME/CFS, fibromyalgia and long COVID, and many B-cell depleting efforts (monoclonal antibodies, plasmapheresis, immunoadsorption, IVIG) are being tried in these diseases.
A small open-label study found that a B-cell depleter called daratumumab (which affects B-cells differently than Rituximab) was either highly effective or did nothing at all. By the end of the study, half the patients reported on physical functioning questionnaires that had “almost no physical limitations”.
While it’s not clear how daratumumab achieved its effects, its significant reduction of IgG4 antibodies suggests that IgG4 antibodies may play a major role in ME/CFS. While the baseline levels of IgG4 antibodies were not high, these antibodies can lead to an overproduction of collagen, resulting in excessive growth of connective tissue. That’s an interesting finding given the collagen thickening in the capillaries found in the Wust group’s recent muscle study, which could be impairing blood flows.
Last month, the authors applied for a 66-patient, randomized, double-blind, placebo-controlled study of daratumumab in ME/CFS.
A smaller Japanese Rituximab study, which will use higher maintenance doses than did the big Rituximab study, is underway. It will feature numerous exploratory analyses in an attempt to understand both Rituximab’s effects and what’s happening in ME/CFS.
Check out the first blog on the 2025 Charité ME/CFS and long COVID Conference
Health Rising’s Quickie Summer Donation Drive is on the Brink!
Thanks to everyone who has brought Health Rising’s summer drive to the brink of success!

After doing some digging – the light bulb went on.
When I first saw daratumumab, I thought, “Oh boy, another B-cell depleting drug – and from Norway! Don’t they know when to give up?”.
But then I started digging. I found that the big Rituximab study may have had some problems (low maintenance dose), that Fluge and Mella had demonstrated that B-cell depletion could be effective, that daratumumab affects different B-cells than Rituximab, and that the pilot study had yielded very good results. Plus, there were all these studies suggesting that something had gone wrong with the B-cells in ME/CFS. FM and long COVID…Now I was excited.
It just took some digging. If that kind of work supports you, please consider supporting us!







Your work gives hope to me and my daughter, who has suffered from moderate to severe ME for the past 8 years. It’s incredibly encouraging to know that research of this kind is making progress – albeit slowly. We’re so grateful for your clear, understandable reports on this research, and the positive tone always promotes more hope.
Thanks so much, Sally. I really appreciate the work Fluge and Mella are continuing to do in Norway. When Rituximab didn’t work out they could have folded their tents but they thought they were onto something with the B-cells in ME/CFS and kept on with cyclophosphamide and now daratumumab. Good for them!
Could not agree more. Well said!
Cort there’s a need of spinal surgery.i have very severe ME/CFS
The cost is 5000$.even a small amount will help
This is so interesting to me, thanks for your report. I’m really happy hearing about the Japanese study. In 2020 after 23 years with ME/CFS I developed Diffuse Large B Cell lymphoma, a blood cancer. One of the drugs I was treated with was Rituximab. After I recovered from the chemo and the cancer, I had 18 months of such good health that I completely forgot I had ME/CFS. I walked long distances every day, did graded exercise, took on all sorts of projects, had no problems with stress.
Yes, I fell over in the end. Almost to the date of two years after the course of Rituximab (10 doses) I had a bad flare that I have not yet recovered from. A couple of other stresses such as having to sell my house, and maybe-or-not relocating to a tropical climate didn’t help, plus the exercise of course … and I’m back to who I was but worse. But, you know, good memories of Rituximab.
Rita, can I ask what your complete protocol was ?
– next to Rituximab There are other chemo-type drugs That can help with ME-symptoms
– e.g. cyclophosphamide
Sure, the complete protocol was R-CHOP …
Rituximab:
This is a targeted therapy drug that specifically targets and destroys B-cells, a type of white blood cell, which are often involved in lymphoma.
Cyclophosphamide:
This is an alkylating agent, a type of chemotherapy drug that damages the DNA of cancer cells, preventing them from dividing and growing.
Doxorubicin:
Another chemotherapy drug, doxorubicin, is an anthracycline antibiotic that interferes with DNA and RNA, also inhibiting cell growth.
Vincristine:
This chemotherapy drug, a vinca alkaloid, works by stopping cancer cells from dividing and multiplying.
Prednisone:
This is a corticosteroid, a type of steroid hormone, that helps to reduce inflammation and also has anti-cancer properties.
Lots of these potential treatments will not trickle down into treatments for many years, and they will be expensive. When I was reading the Treat Me post, I noticed that while IVIG made 64% of the participants feel better, IV saline resulted in 52% of the people feeling better. Maybe we should lobby to get IV saline an approved treatment for these infectious disease disorders as it could help mitigate the suffering of so many with these conditions.
That’s one of the things I repeatedly wrote about. The Rituximab trial failed because the results of both Rituximab and placebo were rather good and the results of Rituximab were only moderately better then those of placebo.
The thing is however that Rituximab in the trial was AFAIK distributed via a saline solution dilution and the placebo in the study was IV saline. And there are (somewhere, too tired to look for references now) studies showing that some protocols of IV saline are all on their own helpfull. I can’t help but feel there is something there waiting for further analysis.
If (some protocols for) IV saline worked and meds plus saline would be better and worth the extra side effects, all the better.
Do you have a reference to a paper comparing IVIG (that most likely is distributed via saline) to saline? 52% improvement for saline versus 64% for IVIG + saline would be fairly close to the results of the Rituximab study.
If either one of those studies would be too small (in number of participants), the difference easily would fall below a P That would put a whole other light on Rituximab, IVIG and IV saline as a treatment. Maybe the biggest find would be that IV saline in its own is a very cheap and effective treatment, since the researchers used it as placebo and barely tried to find the optimal dosing regimen for IV saline. If so, IV saline plus Rituxiomab or IVIG could be optimized in terms of dilution and dose spreading too.
=> Combining those two studies and someone (not me) bringing that to the attention of the authors of both studies could be valuable.
The results are from the Treat Me Open Medicine Foundation results from this blog. It is in the list of things that helped patients in the study along what percentage of the participants were helped by the intervention. https://www.healthrising.org/blog/2025/07/11/treatme-open-medicine-foundation-long-covid-chronic-fatigue/
My daughter who also has ME/CFS is really helped by IV Saline, but it is very expensive in Maui and as a struggling artist, she can only afford it when she really feels bad.
Great information, Cort. Thanks for all you do.
One of the things I remembered when reading your article about Darzalex (daratumumab) is that some individuals who were diagnosed with ME/CFS and/or Fibromyalgia developed multiple myeloma, decades later. Darzalex was developed specifically for multiple myeloma, so I’m wondering what the co-morbidity rate is for those of us who’ve lived with these syndromes for decades, who later developed MM. Are any of the researchers tracking this info?
I know that many researchers outright reject study subjects past 65 years of age. But the vast majority of MM patients are diagnosed after 65; it’s not a disease of youth. Makes me think there’s definitely a connection between the two, given how well Darzalex worked in 6 of those 10 women.
Judi,
I was wondering the same thing. I have MGUS (a precursor to multiple myeloma). I have IgG lamba. When I saw datatumumab mentioned I took notice. I have ME/CFS and Ehlers-Danlos too. Interesting that EDS folks also are not uncommonly affected by disorders like lupus, multiple sclerosis, Sjogren’s Syndrome, rheumatoid arthritis, type I diabetes, Crohn’s disease etc., many of which are connected to the HLA axis in genetics.
Multiple myeloma has many different forms depending on the Ig (IgG, IgA, IgM etc.) involved and what part of the antibody is affected. The “Y” antibody has long chains (the longer part) and short chains (the short part at the top of the “Y”). MM happens when the bone marrow produces too many of a certain antibody and crowds out all others so the body’s immune system is out of balance. There are dozens of drugs being used to treat MM and it is far too complex to discuss here and I probably don’t understand much of it, but I do see some sort of connection to these various problems.
As an aside, daratumumab is not without side effects!
Doctors often do not test for MGUS as it is supposed to not have any symptoms (not entirely true) and has a 1% per year chance of development to MM (also not entirely true). Any of you also have MGUS or Smoldering or MM–and ME/CFS? It would be interesting to know.
Hi, I developed MGUS around 10 yrs ago that morphed into smoldering myeloma around 5 years ago, and I now have multiple myeloma as of the past yr. I’ve been on Darzalex for the past yr. but haven’t noticed significant improvement in ME/CFS symptoms. It would indeed be interesting to know how many ME/CFS folks have developed MM.
“Smoldering? Please explain what
“Smoldering” is?
Roonie, in the most simple of terms, Smoldering is the stage in-between MGUS (a ‘precursor’ stage) and full blown multiple myeloma. People like me get tested periodically to see if our labs become more and more abnormal–which leads to ‘smoldering.’ Eventually the body cannot hold back the changes in the bone marrow (where this originates) and it can become full blown myeloma. There are various lab numbers which indicate this and I am too lazy to look them up. Hope that answers (mostly) your question.
Thanks so much.
I ask because one dr.told me I have a Smoldering infection…but wouldn’t elaborate further…even when I pressed him
Roonie,
If you do have a smoldering infection related to an M-spike as in myeloma, please educate yourself about this condition! A good place to go is http://www.healthtree.org. I don’t know where you are (U.S. or another country), but progressing to myeloma is a very serious thing and you need to get the best care you can find. Kind thoughts your way!
Gez medinger (Youtube) CURED his long covid by fasting as well as many others…
https://youtu.be/5cD3dWuNjh4?si=UTyUiQaiCEro1r3K
I wonder how the overproduction of collagon and its deposition in the blood cells squares with the fact that so many of us develop stretchy skin and seem to have problems with producing collagen?
My daughter just sent me this comprehensive and easy to read article on the potential effects of HHV 6 A and B.
I was really impressed by the information on HHV 6 A.
https://www.verywellhealth.com/hhv-6-and-its-role-in-disease-4156793#:~:text=Human%20herpesviruses%20cannot%20be%20cured,though%20they%20often%20are%20asymptomatic
This sounds promising,. I wonder how they allowed for the effects of dexamethasone on the immune system. I will have to read the study and find out!
Answering my own question (I think): It was a very small amount given so unlikely to have much of an effect.
Both rituximab and daratumabab (darzalex) can be the cure
I have a myeloma and now i am in remission since 4 years. The next treatment i will have when myeloma comes back is daratumabab.
Hi and thanks for giving so much Cort!
Do anyone know if daratumumab have shown any sides effekts for me cfs patients?
Take care!
Rituximab and IVIG helped me tremendously. I agree the low maintenance doses in the trial doomed it.
I have had ME for 26 yrs. I also became sick with MS 3 yrs ago. For MS control, I have had 23 doses of an anti-CD20 drug called Kesimpta (4 doses over five weeks for loading, then once a month thereafter). The ME (severe) has not improved, and I have since been diagnosed with Fibro too. I’m finding the monthly treatments to be seriously butt-kicking ME-wise, so I am considering switching to another option called Ocrevus, as it’s 6-monthly. Ocrevus is also an anti CD20 but works differently (I don’t understand what the difference is, but maybe it does a ‘deeper’ action if it lasts for 5 months longer?). I will definitely let you all know if Ocrevus improves the ME or Fibro. Another option I’ve been offered is a 2 yr chemo regimen using cladribine, which I believe wipes out B and T cells down to the DNA level, so I’m wondering if that’s a similar action to removing “the longer-lived B cells” mentioned in this article? Anyone who loves research and wants to wade in, I’m open to insights, as my ability to research is very impaired due to the brain damage. Thanks x
Great summary of the different treatment trials. Just a quick note: the randomized daratumumab study with 66 patients was approved in early June and opened for recruitment on June 6. Although it’s now underway, it’s not yet fully funded. The Norwegian ME Association is currently raising money for the study through their ME Fund.
I’m the ME Fund’s campaign coordinator, responsible for organizing the fundraising efforts and spreading awareness about the study.
Very good to hear! Thanks Kenneth for keeping us up to date. I hope we can get it fully funded.
Is the study being performed in Norway only? Can those residing in the US take part?
The study is being conducted at Haukeland University Hospital in Bergen, Norway. In theory, there’s no formal rule excluding people living abroad. However, participation requires multiple in-person visits to the hospital in Bergen, so for practical reasons it may be challenging for those residing outside Norway, especially overseas.
Hi,
How would one go about donating to this trial?
Thank you for asking! We truly appreciate Cort Johnson’s work and don’t wish to compete with his fundraising for Health Rising. Since you asked, I’ll just share that all funds raised by the Norwegian ME Association right now go 100% to the daratumumab trial. If you live outside Norway, you can donate via PayPal here: paypal.me/MEnorway. If you live in Norway, you can use https://innsamling360.no/innsamling/gi/142/?&Pk=0&type=1&pane=1&step=1&amt=&returnUrl=%2finnsamling%2fvis%2f142%2f&