

Geoff’s Narrations
The Blog
The GIST

Some long-COVID researchers have met with resistance from large drug companies.
For patients, it all comes down to treatments, and that means clinical trials – and lots of them. Given that, it was hard to read Rowan Walrath’s article, “Long COVID studies stymied by pharma’s lack of cooperation“, in Chemical and Engineering News (C&EN), indicating that big pharma is not embracing long COVID. (Most of the first part of this blog is taken from Walrath’s article. Hats off to her and C&EN for charging to the forefront with three articles on long COVID over the last couple of months.)
For people with ME/CFS, the lack of interest from drug companies is nothing new. Despite the millions of patients with the disease, ME/CFS has seemed like kryptonite to the big drug companies. Except for Rituximab, which was largely patient-funded, and the Ampligen trial done decades ago, it’s hard to think of a single large drug trial for ME/CFS that has taken place. Ampligen is still the only drug that has made it even partway to FDA approval.
Could long-term COVID, even with its tens of millions of patients in the U.S. and the huge potential payday they represent, be suffering the same fate? While it’s clear that’s not going to happen, Walrath’s article indicates that the same biases and problems that have afflicted ME/CFS for decades are hampering the search for a treatment for long COVID.
Avindra Nath’s search for a drug trial is a good example. One might think that Nath—the clinical head of the NINDS intramural center—might have some sway. Nath successfully used a drug called Keytruda to treat progressive multifocal leukoencephalopathy (PML) and has stated he wants to try checkpoint inhibitors in long COVID and ME/CFS. However, his attempt to get MERCK to provide Keytruda, which is used to treat T-cell exhaustion, failed. A similar attempt to get Bristol Myers Squibb to provide a different combination of checkpoint inhibitors met the same fate.
The GIST
- Rowan Walrath’s article, “Long COVID studies stymied by pharma’s lack of cooperation“ indicated that, in some cases, big pharma is not only not embracing long COVID but is unwilling to provide even minimal support.
- After Avindra Nath, for instance, secured the NIH funding necessary to conduct a long COVID clinical trial, two pharmaceutical companies were unwilling to provide the drugs they’re making billions of dollars from every year for free. A similar situation occurred when Northwestern University neurologist Igor Koralnik approached several large drug companies.
- At least three things are stopping big pharma from even attempting to crack what would surely be a huge market for them. Diseases like long COVID and ME/CFS don’t fit in traditional disease categories, no validated biomarkers exist, and the diseases are heterogeneous.
- Biological biomarkers that can track treatment effectiveness would be best. With its initial $1.15 billion outlay, the RECOVER initiative could have taken a deep dive into the molecular underpinnings of long COVID. Instead it chose to spend most of its money using tests most of which have been unrevealing in diseases like ME/CFS. The result – five years later, RECOVER has not fulfilled what should have been its prime mandate: provide biological diagnostic criteria that drug companies could use to carry out clinical trials.
- Symptomatic assessments can work if the FDA validates them, as it did with fibromyalgia. Seth Lederman of Tonix Pharmaceuticals asserts that the quickest way to get big pharma engaged in diseases like ME/CFS and long COVID is for the FDA to tell drug companies exactly what fatigue assessments it can use to get its drugs approved.
- All is not lost, though! While the field may not be moving forward as quickly as we wish, it is moving forward in surprising ways.. Even with big pharma still acting largely allergic to long COVID, many intriguing small and large drug trials are underway. Most of these drugs being assessed have probably never even been contemplated in ME/CFS – a good sign.
- The list of drug trials is long and includes many immune, metabolic, and central nervous system activating drugs (see the blog). Fourteen trials have over 100 participants, and ten have over 200 participants. If successful, some of these trials will surely quickly provide validation and lead to new treatment options for patients.
Here’s the thing: Nath had everything but the drug taken care of. Using the NIH’s investigator-sponsored research funding mechanism, the NIH was willing to fund Nath’s and his staff’s salaries, lab needs, patient recruitment, etc. All Nath needed was the drug company to provide the drug gratis. Nath told Walrath, “All the rest we can do ourselves. We just need the drug.”
While these drugs are not cheap—they cost upwards of 10K a dose for patients – the companies stand to make billions and are already making billions from them. Keytruda earned MERCK and Company a cool $29.5 billion last year. Nath stated, “They make billions of dollars off this drug alone. In dollar amounts, for them, [drug donation] is a drop in the bucket.”
Nath, unfortunately, is not alone. Walrath reported that Northwestern University neurologist Igor Koralnik got the same answer when he approached Eli Lilly, Amgen, and other drugmakers for drugs to treat severe headaches in long COVID. Sounding surprised and rather bitter, Koralnik, who has published a remarkably extensive paper on the neurological manifestations of long COVID, told Walrath:
“It was a big expenditure of energy and time on my end for no results. It would be interesting if you could go to Eli Lilly and can talk to the people who are making the decision about headache and say, ‘Have you ever even heard of Dr. Koralnik?’ I would bet my saddle and my horse that they would say no.”
Nancy Klimas’s attempt to find a company to provide a drug that was: a) no longer being used; and b) just sitting on a company’s shelf, presents another cautionary tale. Klimas was ultimately successful, but it took work. Even after getting remarkable results in her long-COVID patients, Klimas was still turned down by three large drug companies (GSK, Lilly, Regeneron) before she found one willing to help. Klimas called it a “happy ending to this story instead of a terminally frustrating one”.
What’s Going On?
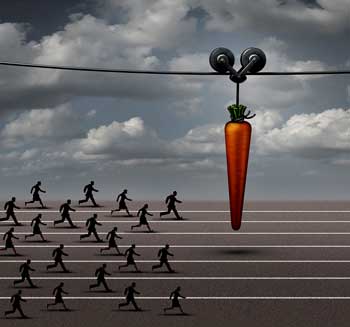
Tens of millions of long-COVID patients in the US alone have not been enough to entice many big pharma companies to provide support.
The big question is why big pharma is not chomping at the bit at the huge potential payday that will clearly result from producing the first FDA-approved drug for tens of millions of people in the U.S. alone.
Walrath’s article suggested that one reason may be quite mundane—and that’s bedeviled ME/CFS at the NIH and elsewhere for decades. It’s a matter of location. Where do you fit a disease that affects so many systems? If you’re a major pharmaceutical company, do you give long COVID to your cardiovascular, metabolic, or immune teams?
Indeed, a general lack of clarity about the disease itself, plus the sheer difficulty in assessing a drug’s effects, appear to be the primary culprits. A biological biomarker that could be used to track drug efficacy would solve all problems. It was that kind of biomarker that quickly turned around drug development for HIV/AIDS.
A biological biomarker is not always necessary, though. No biological biomarkers have been found for fibromyalgia (FM), but FM has 3 FDA-approved drugs and regularly receives clinical trials. FM has something, though, that both ME/CFS and long COVID lack—a clear FDA path to drug approval. Once the FDA stated it would accept validated pain assessments as proof that a drug works in FM, the drug companies quickly came on board.
Because we know much more about pain pathways in the body than we do about fatigue, FM’s path was easier. We know, for instance, that pain-producing pathways, involving nerve centers in the body, the spinal cord, and at least six regions in the brain, as well as at least six neurotransmitters, have been dysregulated in FM. Plus, assessing pain sensitivity levels using objective markers of heat, pressure, and cold is simple.
Nothing close to that has been achieved with regard to fatigue in ME. Numerous validated fatigue assessments do, however, exist. Seth Lederman of Tonix Pharmaceuticals asserts that the quickest way to get pharma involved in ME/CFS and long COVID is simply for the FDA to tell pharmaceutical companies that their drugs will get approved if they meet certain fatigue criteria.
Missed Opportunities – the Biomarker Issue
The clinical trials dilemma the ME/CFS-like subset of long COVID is in could have been predicted. A new, heterogeneous disease characterized by two mostly mysterious symptoms (fatigue and post-exertional malaise symptoms) was never not going to make it with big pharma on symptoms alone.
The NIH’s $1.15 (now $1.65) billion long-COVID RECOVER Initiative presented the best possibility for finding a biomarker. It had the funds to do a deep dive into the molecular underpinnings of long COVID but chose not to. One could understand RECOVER being wary about doing that, but in retrospect, what other choice did it have?
Anyone looking at ME/CFS realized that ordinary laboratory tests would have been largely unrevealing, yet RECOVER has spent much of its money attempting to characterize long COVID using these tests. Five years later, RECOVER has not come close to accomplishing what arguably should have been its first task: producing clear and accurate diagnostic criteria that paved the way for clinical trials.
Not All is Lost

Many drug trials are underway, though, and some are large enough to provide instant validation.
All hope is not lost, though. The field may not be moving forward as quickly as we wish, but it is moving forward in surprising ways. Even with big pharma still acting largely allergic to long COVID, many intriguing small and large drug trials are underway. ME/CFS patients note that most of these drugs have probably never even been contemplated in ME/CFS.
Note that this list focuses on drugs, and because most of the data comes from the https://clinicaltrials.gov/ website, it is surely incomplete. It does not, for instance, include the next $500 million round of long-COVID clinical trials produced by the newly revamped treatment arm of the RECOVER Initiative.
Check out the wide variety of drugs being explored.
Immune Drugs
Antivirals
- Truvada trial (tenofovir disoproxil/emtricitabine, TDF/FTC (Group 1) or Selzentry (Maraviroc) (Group 2) – two repurposed HIV drugs. PolyBio study. Putrino, Iwasaki and Proal (n=90)
- Ensitrelvir – small antiviral trial at UCSF led by Michael Peluso that will explore efficacy and try to understand what’s going on in long COVID
- Remdesivir (Veklury) – antiviral; Remdesivir is being explored in COVID-19, but it’s a broad-spectrum antiviral that may be helpful with many RNA viruses (but not herpesviruses, which are DNA viruses). (Two trials are underway: n=48 and n=150)
- Plitidepsin inhibits coronavirus replication and other viruses, including MERS, Zika, herpes simplex, etc. Has not been tested against EBV or HHV-6, but its mechanism of action suggests it could be helpful (n=90).
Monoclonal Antibodies
- Monoclonal antibody AER002 – small monoclonal antibody trial at UCSF led by Michael Peluso, which will explore efficacy and try to understand what’s going on in long COVID; (n=30).
JAK 1 / 2 Inhibitor
- REVERSE LC – Baricitinib may help with inflammation, neuroinflammation, and blood vessel functioning; (n=500).
Blood Filtration (Apheresis)
- Seraph 100 – apheresis filtration trial attempting to remove pathogens (viruses, bacteria), toxins, and pro-inflammatory cytokines (e.g., IL-6, IP-10) from the blood using heparin; (n=100).
Other Immune
The REVITALIZE Trial – a major effort by a non-profit that will start with upadacitinib (Rinvoq) or pirfenidone and then add other drugs on as the trial progresses; one person with severe ME/CFS experienced an astounding recovery using Rinvoq n=348).
Learn more about the Schmidt Center and its long-COVID work.
- IVIG – RECOVER Initiative – finally a large IVIG trial! (This is part of RECOVER’s autonomic clinical trials, including Ivabradine; n=380). May help with many factors including autoimmunity, inflammation, immune modulation, viral clearance and neuroprotection.
- Selzentry® (maraviroc) plus Lipitor (atorvastatin)—Bruce Patterson is putting his money where his mouth is with a 32-week randomized, 252-patient, double-blind, placebo-controlled, multicenter study.
- Apabetalone is a bromodomain and extraterminal (BET) protein inhibitor that has dual antiviral and anti-inflammatory mechanisms, and may help with blood vessel functioning (n=200 people with long COVID and type II diabetes).
- Autologous stem cells – stem cells can modulate and even rebuild the immune system (n=20 and n=12).
- More UCSF trials! – More small trials have been promised by Michael Peluso to explore efficacy and try to understand what’s going on in long COVID
Metabolism and Mitochondria
- Rapamycin– an MTORC1 inhibitor is such an intriguing possibility. Rapamycin can improve mitochondrial functioning, regulate immune senescence, reduce inflammation, and rebalance the immune system. Anecdotal reports suggest it can be helpful in ME/CFS, and a recent journal article proposed that long COVID and ME/CFS are MTORC1 syndrome diseases. Two trials (Simmaron– n=150; Mt Sinai – Iwasaki and Putrino – size unknown).
- Bezisterim – a safe drug that may be able to reduce neuroinflammation, and help with metabolism (insulin sensitization), (n=208).
- Metformin – metformin has been shown to reduce the incidence of long COVID, may positively enhance metabolism, gut functioning, and have anti-inflammatory and antiviral effects. Two trials – one small (n=16) and one large (part of the REVIVE trial) (n=1,500).
- Dapagliflozin – could reduce inflammation and improve metabolism – currently used in type 2 diabetes (n=192).
Central Nervous System
- Amantadine, a dopaminergic, NMDA receptor antagonist, and mild antiviral, may help with fatigue and brain fog. Two trials (n=30 and n=60)
- Ivabradine – may be able to reduce heart rate and improve autonomic functioning trial (part of RECOVER autonomic clinical trials, including ivabradine; n=380). Plus another 250-person POTS/long-COVID trial
- Fluvoxamine Maleate 100 MG – an SSRI, fluvoxamine may help with ER stress, reduce inflammation and neuroinflammation, and protect the blood-brain barrier; part of REVIVE trial (n=1,500)
- Ketamine – may help with neuroinflammation and improve cellular energy production; n=20)
- DAOIB for the Treatment of Cognitive Impairment Induced by COVID-19 – an Open Trial (n=40); unknown drug
- RISE Project – Budesonide/Formoterol; Vitamin C combined with Coenzyme Q10 oral treatment; Montelukast tablets oral treatment; inhaled corticosteroid (budesonide) reduces airway and systemic inflammation. Montekulast may help with breathing, reduce neuroinflammation, and have antiviral effects (n=632).
Coagulation
- Lumbrokinase—an anticlotting and anti-platelet hyperactivation agent, lumbrokinase could reduce inflammation and improve blood flow. PolyBi- supported ME/CFS, long COVID, and Lyme study at Mt Sinai; size unknown.
Gut
- Larazotide (AT1001) – restores the barrier function of the intestinal lining and reduces abnormal permeability, often referred to as “leaky gut”; (n=48).
Conclusion
Yes, many big pharma companies are not embracing long COVID, but not all hope is lost. Investigators are clearly interested in long COVID, and clinical trials employing a surprisingly wide array of drugs are underway. Many of these drug trials are of a scale that is essentially unheard of for ME/CFS. Fourteen trials have over 100 participants, and ten have over 200 participants. If successful, some of these trials will surely quickly provide validation and lead to new treatment options for patients.






Thanks Cort! It’s actually quite a decent number of trials, far far more than we ever had with ME/CFS. Here’s hoping. It gives me at least a bit of hope.
You mention in the article that Nancy Klimas has had great results with long covid patients. ‘Great results’ sounds almost too good to be true. If so…. What is she using, and what are next steps?
Actually, ‘remarkable results’!
She used monoclonal antibodies developed for the early strain of the coronavirus. THe results in the case study were remarkable – full and rapid recoveries of several people. Michael Peluso is study mab’s developed for later stages.
She has probably begun the larger trial by now.
Why has nobody but Dr. Paul Cheney and the HHV-6 Foundation looked at artesunate as a safe and effective antiviral?
https://hhv-6foundation.org/research/hhv-6-antiviral-drug-resistance
Table 1. Current & Phase III drugs with activity against HHV-6
.
Artesunate Malartin,Artesor malaria Excellent Yes Minimal
This is an antimalarial that was developed in 1977 but not approved for use in malaria in the U.S. in 2020. Dr. Cheney used it in ME/CFS patients because Artesunate also exhibits strong antiviral properties.
Of course, no pharma could patent it so there may explain the loss of research interest.
In case anyone wants to try it, it is relatively safe in over the counter, herbal form. It’s used frequently by people with tick borne diseases, here, Babesia. https://www.treatlyme.net/guide/kills-babesia-a-brief-guide
“Artemisinin 100 mg is an herbal medication. Start at 2 or 3 pills 2 times a day for 3 days on the medication, then take 11 days off. Continue this 14-day cycle. The goal is to reach 5 pills 3 times a day on the 3 days the medication is taken. I use artemisinin this way to overcome resistance. Be aware, the dose on the 3 days is quite strong. Often it causes a worsening of the Babesia symptoms beginning on the second day and sometimes lasting until six days later. If a person is very medicine sensitive, I start at 1 or 2 pills 3 times a day.”
“Artemisinin resistance can occur for two reasons. First, the liver learns to clean artemisinin out more quickly after a person is on it for a few days. The other mechanism could be like what happens in malaria. In malaria, partial resistance develops in the germ after a few days. In malaria, it is shown that artemisinin works best by pulsing on and off the drug like I recommend above.”
I am not entirely surprised that even a high profile researcher like Nath couldn’t get Keytruda from MERCK even with everything ready to go and paid for by NIH except the drug itself.
If MERCK went with it, they would sort of indicate that their money maker, now costing 10000$ a dose, could be provided for 10000$ or less a year to provide large amounts (millions of patients times 365 daily doses a year). Even if the studie’s outcome would be negative, they sort of would have indicated they believe that is feasible and insurrers might put extra pressure on them to lower prices. That 10000$ a year is my upper estimate of what insurrers would be able or willing to pay for a drug used by millions of long covid patients.
One could say 10000$ a year times millions of patients would be a lot of money. But if they made 29.5 billion on it last year, that’s equivallent to 2’950’000 paying 10000$ for it for treating cancers, or less patients if they need multiple doses. Either way, if they sold all doses at the same price, to cancer patients and long covid patients alike (if they didn’t drastically decrease their price per dose many would try to get a second diagnosis for long covid next to their cancer or buy it off the hands of long covid patients), they would need far more then 3 million long covid patients buying their drug to come close to break even. The production cost to produce so many doses would take a far larger percentage of the dose price then it is today. That is on top of their need to fund several expensive phase X trials with uncertain outcome.
On top of that, they and other farmaceutical companies have many similar very expensive monoclonal antibody drugs for specialized purposes. The price of all those could come under pressure if they demonstrated that they can be produced profitable at a fraction of the cost. At the very least regulators and insurrers would like to have a look at their price structure. And that “all” for a drug that may or may not be supplanted within years by cheap to produce out of patent drugs like Metformin or rapamycin if they’d prove equally effective.
In my opinion: forget those expensive in patent drugs, even if there would be a huge market for cheaper doses more then making up for the loss in high prices they hold now. From a corporate profit point of view they are not stupid nor ignorant.
Keytruda can be a life-saving medication in certain conditions like some inoperable cancers but it is not without serious side-effects. You can only stay on Keytruda up to five years because of the risk of developing serious auto-immune diseases which can be lethal.
Voici la traduction avec un ton naturel et interrogatif :
“Is it possible that they’re trying to prevent certain known but previously downplayed side effects from resurfacing during clinical trials?”
“Translated from french by ChatGPT”.
Be very careful trying any of these options w/o knowing how to do it properly! Start Low dose and go slowly is the key to not getting very sick!
#1 Montelucast has a black box warning! By day 3 of taking it I was very very depressed. Tried again a month later and within a day the depression was back.
#2 You can try Artemesia yourself but it has Very specific directions.
Details here: https://www.treatlyme.net/guide/best-herbal-antibiotics-for-lyme-bartonella-babesia ” Artemisinin 100 mg 2 or 3 pills 2 times a day for 3 days on the medication then take 11 days off. Repeat this 14-day cycle, increasing the dosage each cycle. The goal is to reach 5 pills 3 times a day on the 3 days the medication is taken. I use artemisinin this way because the intestines develop an enzyme that destroys this herbal medicine if it is used longer than three days. Be aware the dose on the 3 days is quite strong. Often, it causes a worsening of the Babesia symptoms beginning on the second day and sometimes lasting until six days later. If a person is very medicine sensitive, I start at 1 or 2 pills 3 times a day.” Didn’t work for me but everyone is different.
#3 Directions/info on Lumbrokinase at the same website as well as a lot of other helpful info on recovering from chronic diseases.
#4 Fibro pain decreases after changing gut microbiome. You can do this yourself by changing your diet, taking herbs and supplements and the “right” probiotics. Start by finding a Dr (likely an ND) to place an order with Genova Diagnostics to find out what exactly you need to work on. Not too expensive and will save you a lot of money in the long run. Use the results to make changes. And please, please avoid social influencers online! The number of quacks with fad diets designed to make them money and you broke it sky-rocketing. There is a special place in hell for these people taking advantage of sick people to make money. Dr Ross, above is a real DR in WA and his info as well as Cort’s have me back to a functioning life. 🙂
Cort, thank you very much for this update. Maybe I’m naive, but all things considered, the situation looks quite promising to me. Many (most?) of these trials are with drugs/therapies that are already available. So if even just one of these trials proves to be effective, we patients have a viable treatment option available “off the shelf” (insurance coverage is another matter, of course!).
Is it unreasonable to hope or even expect that one of these treatments will be effective?
Yes! I was surprised at how much work was actually being done and am optimistic as well. Just one big treatment trial success and we have more options.
Hello Cort,
It’s “champing at the bit”, not chomping.
https://www.phrases.org.uk/meanings/champ-at-the-bit.html
Finally made my donation. Sorry I was a bit late this time.
Cheers,
Sarah T
Champing! Thanks for that and the donation! Never late – always appreciated 🙂
And Lilly is currently running a “Get Better” ad campaign that emphasizes the company’s “commitment” to improving health outcomes and reducing disparities in access to care. A total load of bull when they can’t be moved to help the millions of MECFS sufferers.
The root issue is sticking to a too simplistic model for the condition and treatment. “One treatment treats them all” is the stuff of fantasy writers (and Pharma Marketers).
Looking at dozens of microbiome from ME/CFS and Long COVID will see a consistent pattern of dysbiosis in the microbiome. Unfortunately, each is different and an antibiotic that helps one can make a different one worse. Come of Cecil Jadin recommended antibiotics are very frequently recommended in adjusting the microbiome.
You will not see success doing one static protocol for all people with these conditions. Each person needs their own custom protocol that will likely need to be adjusted every few months based on how their microbiome drifts to recovery from each intervention.
This is why the expert system at MicrobiomePrescription.com was built – to allow microbiome-aware treatment plans.
“The root issue is sticking to a too simplistic model for the condition and treatment. “One treatment treats them all” is the stuff of fantasy writers (and Pharma Marketers).” – Agreed. There must be many roads that lead to Rome with these illnesses.
I don’t know of you saw but a study just found that giving mice fecal microbiota from FM patients turned them into FM’y mice.
https://pubmed.ncbi.nlm.nih.gov/40280127/
Yes I saw. The key bacteria identified ” Since mice transplanted with the FM gut microbiota showed reduced levels of specific bile acids (e.g., ursocholate) and bile-acid-metabolizing bacteria (Lachnoclostridium scindens), ”
Full Text: https://www.cell.com/neuron/fulltext/S0896-6273(25)00252-1?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627325002521%3Fshowall%3Dtrue
So two things are needed: Increase bile acid (supplements) and increase Lachnoclostridium scindens. The substance known to increase this bacteria include:
High-fibre diet {Whole food diet}
Rheum × hybridum {Rhubarb} & Rhubarb x Peony {Rhubarb Peony Decoction}
bacillus subtilis {B.Subtilis }
Coffee (Caffeine)
From https://microbiomeprescription.com/library/details?taxon=29347
Note that 80% of items listed on that page DECREASE it. It is very important to reduce or eliminate those substances in the short term.
I just did an in depth review of their hypothesis
https://cfsremission.com/2025/05/04/reviewing-recent-study-on-fibromyalgia/
I recently read an article that MEcfs researcher, Ian Lipkin, at Columbia University, was in talks with a pharmaceutical company before his funds got cut off by Trump. Does anyone know what drugs were being considered and/or if the funds have been restored?
I’m trying to get an interview with him. 🙂
Wow excellent! Fingers crossed!!
Do we need the pharmaceutical companies to give the drug? Aka do we need their permission or can we raise the money and just buy it for a small trial?
Has anyone thought to study Cyclophosphamide? I did chemo last year and my Long Covid dysautonomia & PEM symptoms were gone during treatment. My Oncologist said it does something to T-cells. I guess its used as a low dose for autoimmune disorders sometimes.