Geoff’s Narrations
The Blog
Big studies like this recent preprint, “Persistent immune dysregulation and metabolic alterations following SARS-CoV-2 infection, take a village. One nice thing about this village was how widely collaborative it was. Led by NIH researchers with help from the mighty LIINC long COVID project at the University of California, San Francisco (UCSF).

With people who were never exposed to the coronavirus, people who recovered from it, and long-COVID patients participating, it was a most interesting study, indeed.

It took a village, indeed, and no wonder – the study did “comprehensive cellular and transcriptional immunometabolic profiling”. (To be sure, there’s no way a study can be truly comprehensive – there are just too many possibilities now – but this study did a lot.)
It examined:
- Inflammatory biomarkers – interleukin (IL)-1β and IL-18, tumor necrosis factor-α (TNFα), interferon-gamma-induced protein 10 (IP-10), soluble urokinase Plasminogen Activator Receptor (suPAR), and eotaxin
- Mitochondrial superoxide assay (inflammation)
- Lipid peroxidation assay (inflammation)
- Intracellular GSH levels (antioxidant)
- Did a non-targeted metabolomics analysis, i.e., an open-ended analysis.
- Flow cytometry immune cell typing – assessing the types of immune cells present
- Single cell RNA sequencing analysis – (small group) – the most precise kind of gene expression analysis
- DNA methylation – epigenetic analysis.
These complex, multi-systemic studies are what the NIH excels at, and let’s hope none of these researchers have been affected by the funding cuts at the NIH.
Somehow, LIINC found 30 people who had never been exposed to the SARS-CoV-2 virus and then contrasted them to 38 people who recovered from COVID-19, and 58 people with long COVID.
The GIST
-
Being infected by some viruses can result in long-term changes that remain after recovery.
This large, complex long COVID study did what no other study has done before: it compared people who had never been exposed to the coronavirus with people who’d recovered from it, and long COVID patients.
- This is an important comparison because studies have shown that some viruses trigger radical alterations of the immune system long after they’ve been vanquished.
- The study found simply having a COVID-19 infection – even if a person had fully recovered from it – produced dramatic and quite familiar changes. In several ways, recovered COVID-19 patients looked very much like long-COVID patients (!) – but were very different from the people who had never been exposed to the coronavirus.
- The recovered COVID-19 participants displayed “remarkably decreased” tryptophan levels, hyperactive monocytes, high levels of inflammatory lipid byproducts, altered metabolic gene expression, activated inflammasomes, increased T-cell activation, and oxidative stress.
- All of these have been found in long COVID and/or ME/CFS patients – yet these people were perfectly healthy.
- Long COVID patients exhibited even further drops in tryptophan, providing additional evidence of inflammation, inflammatory lipids, and oxidative stress.
- It took the immune findings, though, to really differentiate the long COVID patients from the recovered COVID-19 participants. “Distinct biological processes” indicated that a sustained T-cell activation was probably leading to T-cell exhaustion. Some T-cells were in a senescent, dysfunctional, and inflammatory state, characterized by aging.
- Plus, numerous downregulated pathways involving monocytes suggested they were stuck in an immature, naive state. The authors reported, “This confirms a profoundly altered myeloid profile in LC.”
- With both arms of the immune system (innate (myeloid)/adaptive) affected, an AI Perplexity analysis of the results concluded that they indicate that a “profound immune dysregulation with implications for infection control, inflammation, and disease progression…(reflecting) a shift toward immune tolerance or exhaustion.” had occurred.
- Regarding treatment, authors reported that the viral persistence, immune activation, mitochondrial dysfunction, and metabolic derangements seen all represented potential treatment targets.
- They suggested supplements (Vitamin C plus L-Arginine) and immune modulators, such as baricitinib (a major study is underway), to combat oxidative stress. Additionally, they recommended the use of MTOR-inhibiting drugs, such as rapamycin and rapalog, to regulate cellular senescence, enhance mitochondrial function, and reduce inflammation.
- All in all, the findings indicate that a COVID-19 infection has a profound impact on several bodily systems, even in individuals who have fully recovered from it. What tips COVID-19 over into long COVID is not clear, but this study suggests that immune dysregulation could play a key role.
Please Support Health Rising and Keep the Information Flowing
Results
Winnowing the Wheat From the Chaff…

Being infected with certain viruses can lead to long-term changes in the immune system.
The fact that the study included healthy controls who had not been exposed to COVID-19 gave the researchers the opportunity to contrast them with healthy people who’d come down with COVID-19 and then recovered.
This is an important question because we know that being exposed to a virus, whether one recovers or not, can have profound effects on the immune system with certain pathogens. Cytomegalovirus infections, for instance, leave a particularly strong lasting imprint on the immune system even in those who recover from them. Simply being infected with the virus reduces immune diversity, accelerates immune aging, and promotes chronic inflammation over the long term.
People with long COVID may be similar in kind but different in degree from recovered COVID-19 patients; that is, they both could exhibit similar biological abnormalities, but the abnormalities are more severe in the long-COVID patient. Alternatively, long-COVID patients may be different in kind, meaning that something distinct happened to them as a result of being exposed to the virus.
It turned out that both were true. In some ways, people with long COVID looked very similar to those who recovered, and in some ways, they were distinct.
(Complicating the picture a bit in this study is that the researchers sometimes (several epigenetic tests, immune exhaustion markers, mitochondrial ROS, lipid peroxidation, plasma biomarkers) either put both the recovered and long-COVID patients in the same basket (called COVID-19) or did not show the long-COVID group results.)
Kissing Cousins? The Recovered COVID-19 and Long-COVID Patients.
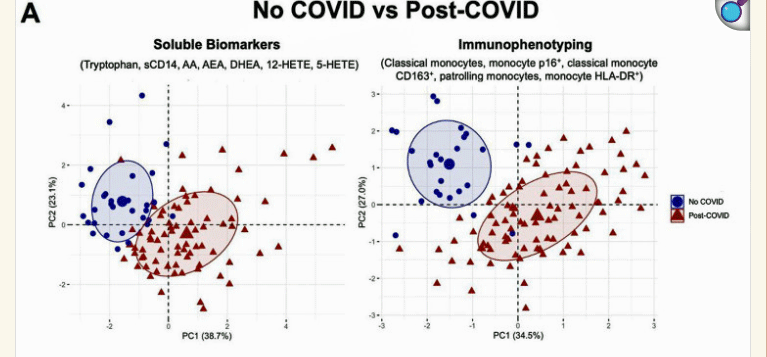
Look at the beautiful separation in the soluble biomarkers and monocytes between the people who were never exposed to COVID and those who were.
Talk about strange relations. Simply having a COVID-19 infection – even if a person was fully recovered from it – produced dramatic and quite familiar changes. In several ways recovered COVID-19 patients looked very much like long-COVID patients (!) – but were very different from the people who had never been exposed to the coronavirus.
In short, some of the processes that lead to long COVID persisted in recovered patients.
The recovered COVID-19 group had “remarkably decreased” tryptophan levels, hyperactive monocytes that are spitting out inflammatory mediators (sCD14/sCD163), high levels of inflammatory lipid byproducts, altered metabolic gene expression, activated inflammasomes, higher caspase-1/4/5 activity and plasma IL-1β levels, increased T-cell activation, and increased oxidative stress.
Despite being well, their systems had undergone tremendous changes. One wonders if some of these people are being set up for a case of long COVID / ME/CFS when some other stressor tips the balance against them. All the above abnormalities have been found in ME/CFS and/or long-COVID patients.
In some of these results, though, a difference in degree is found. Long-COVID patients presented with an even further drops in tryptophan, a significant association between MitSox (oxidative stress) and intracellular levels of a regulatory molecule called p16INK4a that tamps down oxidative stress was found, and so were elevated suPAR levels (associated with increased inflammation, tryptophan degradation)
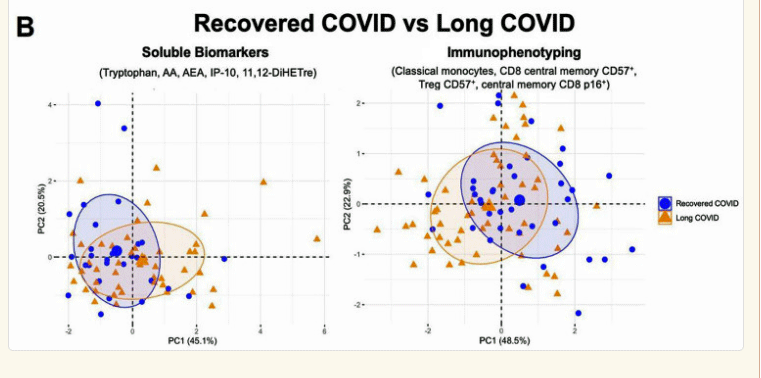
Same tests, but notice how little separation we see between the recovered COVID-19 patients and long-COVID patients.
The “drastic” tryptophan decrease in the long-COVID patients got the authors’ attention. They attributed it to persistent gut damage caused by the coronavirus, as well as the increase in arachidonic acid (see below) that was found. Problems with tryptophan metabolism have been a focus of research in ME/CFS and fibromyalgia for years.
A regression analysis using the lipid findings provided additional insights into long-COVID patients. Lipids are fatty compounds that play important roles, well, almost everywhere, including energy production, many intracellular processes, and cellular signaling. They make up much of the cell’s membranes, which means they’re exposed to virtually everything the cell touches and are at particular risk from oxidative stressors. They’re also particularly abundant in the nervous system and brain.
With reduced levels of two “good” lipids that are associated with beneficial cardiovascular, neurological, and anti-inflammatory effects, the recovered COVID-19 patients weren’t exactly in great shape lipid-wise, but at least they weren’t hurting. Increased levels of two pro-inflammatory lipid byproducts – arachidonic acid and 11–12-DiHETrE in the long-COVID group, on the other hand, indicated that the long-COVID patients were.
At high levels, arachidonic acid is a nasty piece of work. Liberated from cells that have become injured (think free radicals tearing little chunks out of the cellular membranes), it’s metabolized via a variety of pathways into several inflammatory mediators, including prostaglandins (inflammation, pain, and fever), and leukotrienes (inflammatory mediators). (Wirth/Scheibenbogen believe high levels of prostaglandins and bradykinins are wreaking havoc in ME/CFS patients’ blood vessels.)
In all, it seemed like long-COVID patients might have more inflammation, oxidative stress, and lower tryptophan levels than the recovered COVID patients. Was that enough to tip someone with COVID-19 into a long-COVID patient?
Perhaps. We hadn’t gotten to the T-cells or the monocytes yet, though.
Dysregulated Immune System, Indeed
Things started to pop out when the researchers got to the T-cells.
Only in the T-cell analyses did the long-COVID patients really begin to pop out. Elevated p16INK4a and killer-cell immunoglobulin-like receptors (KIRS), and lower CD57 expression in one CD8 T-cell subset suggested they were approaching senescence; i.e., were in a “dysfunctional, aged state”. Not only are these T-cells less able to find and attack pathogens, they’re also highly pro-inflammatory. (Ouch)
A small, single-cell RNA sequencing (gene expression) analysis showed once again how important single-cell analyses are in these diseases. It indicated that the T-cells in the long-COVID patients displayed “distinct biological processes”, indicating that sustained T-cell activation (leading to exhaustion?) was present.
Similarly, numerous pathways involving monocytes (endocytosis, response to external stimuli, regulation of inflammatory responses, cytokine-mediated signaling pathway, and myeloid activation) were downregulated in the long-COVID patients. The authors reported, “This confirms a profoundly altered myeloid profile in LC.”
The two findings were of a pair: monocytes tended to be immature and lacked the ability to surveil pathogens, while the T-cells appeared to be aged, highly activated, and headed for senescence and exhaustion. Now we really had something.
Their conclusion that the innate and adaptive immune systems were not playing well together jives with many past findings, including Nath’s intramural ME/CFS study.
“Our data suggest that an unbalanced innate and adaptive immune recovery following SARS-CoV-2 infection leads to a senescent phenotype with impaired CD8 function, potentially contributing to LC symptoms.”
AI Perplexity’s Take
Of course, I had to ask AI Perplexity what it thought about this. When asked about the significance of these immune findings, AI Perplexity was on the same track. It reported that they indicated a:
“profound immune dysregulation with implications for infection control, inflammation, and disease progression…(reflecting) a shift toward immune tolerance or exhaustion. Furthermore this “imbalance is central to pathologies like cancer, diabetes, and sepsis, highlighting monocytes as key therapeutic targets for restoring immune homeostasis.”
Treatment
The authors reported that the viral persistence, immune activation, mitochondrial dysfunction, and metabolic derangements seen all represented potential treatment targets.
Regarding the increased oxidative stress and inflammasome activation, they suggested supplements and immune modulators such as baricitinib (big long-COVID study underway) that act upstream of the inflammasome and other inflammatory pathways. (They noted that herpesvirus reactivation or increased levels of coagulation could limit the use of some treatments.) One survey study found that combining L-Arginine with vitamin C (2 vials/day of L-Arginine 1.66 g + 500 mg of liposomal Vitamin C) might be helpful. (A blog is coming up on natural anticoagulants.)
The authors also called for assessing immunomodulators “which can regulate autophagy and cellular senescence, and influence arachidonic acid levels, and have anti-tumor activity but will not reactivate the herpesvirus”: That seemed like a tall order indeed, but rapamycin and other mTOR inhibitors, though, fit that bill and several ME/CFS and long-COVID studies are underway.
When asked, “Which mTOR inhibitors can regulate autophagy and cellular senescence, influence AA metabolism, and have anti-tumor activity without reactivation of herpesviruses?”, AI Perplexity agrees with Rapamycin/Rapalogs and added mTORC1/mTORC2 Kinase Inhibitors such as Torin1, AZD8055 and second-generation mTOR-KIs with Improved Selectivity (e.g., RapaLink-1).
The authors don’t mention them, but some diets (keto, protein restriction, fasting) as well as supplements (resveratrol, curcumin, quercetin, EGCG, berberine, caffeine, omega-3 fatty acids, r-lipoic acid), have, in high concentrations, in preclinical studies (not human trials), inhibited MTOR.
Conclusion
All in all, this most interesting study uncovered a couple of surprising things. Whether healthy or still ill, the brush with the coronavirus left quite a mark. Many of the same processes (very decreased tryptophan, monocyte activation, high levels of inflammatory lipid byproducts, altered metabolic gene expression, increased T-cell activation, increased oxidative stress) believed to underlie long-COVID pathology are present in recovered COVID-19 patients as well.
In some cases, such as tryptophan reduction and inflammatory processes involving lipids, the pathology appears to be significantly more severe in people with long COVID.
Just what tipped people with COVID-19 into long-COVID patients wasn’t clear – and it still isn’t – but the immune cells analyses, and in particular, the single-cell gene expression tests, highlighted some powerful immune differences. Overly activated and aged T-cells paired with immature monocytes suggested that a profound immune dysregulation present in long COVID was not found in the recovered COVID-19 patients.
That suggested that more profound immune dysregulation could be the straw that breaks the camel’s back in these diseases. On the treatment side, it was good to see baricitinib, metformin, and rapamycin show up again.





Interesting, VERY interesting if the study would be confirmed. Looking at the graph separating non-Covid-infected controls from Covid-infected controls and then comparing it with the graph separating recovered-Covid from long-Covid patients it resembles that the first graph shows the bigger and clearer / cleaner separation.
Confirmation would put a HUGE dent in the “if the infection is over and the virus is eliminated (or at least undetectable), then there “scientifically” can’t be anything biologically wrong with you. Such claims would de facto become the unscientific nonsense rather then patients’ claims that they remain ill despite no viral markers nor organ damage to be found.
Looking at above graphs, I would go even further: post-viral “condition” is the de facto situation after Covid infection. Regardless of recovering, the immune changes are long lasting and profound in all people who got infected. This change is probably even more profound then the immune change between recovered and non-recovered patients, except for the all important lingering load of symptoms in long-Covid that is. That again says: post viral immune alteration is a huge thing in long-Covid and probably many more illnesses.
Cytomegalovirus infections were already shown to leave a profound lasting immune alteration according to the link in the blog, but at least those virusses persist life long in the body. That is just as their cousin Epstein-Barr-Virus.
The ramifications for medical science and long-term us patients could be huge. Potentially every (strong or not even so strong) infection long time alters our immune profile. Combined with our genes and evironmental influences each infection could lead to a divergence in immune state, each possible to trigger long term health alterations *not only due to the infection but even long after the infection is cleared*.
Cancer research, research into auto-immune conditions, metabolic conditions, mental conditions, aging… all could be profoundly influenced by the the set and timing of infections we lived through including the many small infections we overcame easily. It sounds like this “set of
infectious history” might be a rather large part of the epi side of the epi-genetic influence on our health. With it, better understanding and curing these illnessess would require… in depth studying of long lasting post viral immune alterations.
Thinking of it. Likely the immune response during the infection plays a great(er) role in the long term post viral alteration then the exact pathogen itself.
IF so: many food problems are immune based. Allergies certainly are, many intolerances IMO quite likely are. Over a long time, the loads of antigen should be much higher then the loads of many infections. That is: when having unknowingly eaten something you are intolerant for for years or decades, the total cumulative mass of that allergen you have eaten has a good chance to be much higher then the cumulative mass of a specific virus or bacteria you had to deal with when infected.
An immune response to an allergen basically is (very close to) a largely harmless substance being mistaken for a pathogen. By that reasoning, each long lasting contact with food you are intolerant or allergic to has IMO a large chance to profoundly alter your immune system even long after you stop consuming that product. So just stop eating the products so often doesn’t make you healthy again, as so many of us experienced. A rather difficult to understand and long lasting memory of the past problem is sort of imprinted on your immune state. That memory alone could tell the body to inhibit out of safety as it “remembers” having encoutered many difficult problems in the past before.
“A rather difficult to understand and long lasting memory of the past problem is sort of imprinted on your immune state. That memory alone could tell the body to inhibit out of safety as it “remembers” having encountered many difficult problems in the past before.”
I think you’re touching on something very important here. A smart immune system never forgets. It remembers. In my case, it remembers the assault on my gut that long-term use of antibiotics caused, and the candidiasis and bad bacteria that flourished as a result. Even though my gut is better, my immune system is still on a hair-trigger, ready to go into hyper-drive if I swallow anything that could pose a threat, even if it’s just a banana.
Great points! I remember Nancy Klimas saying “pathogens are back”…Who knows how big of a role they play in many, many diseases.
Remarkable LIINC found 30 people who had never been exposed to the SARS-CoV-2 virus, You would think that after 5 years of corona everyone would have been infected. That said I would also like to see a separation in the research groups between vaccinated and unvaccinated against corona. It is known that vaccinated people can have persistent immune problems.I also wonder how long these people are sick. It seems that t-cell exhaustion occurs later.
I’m glad there are researchers continuing long covid research. But I don’t think there is much difference between a covid virus and any other random viruses in the past. I developed dysautonomia-POTS from a generic virus in 1994. The symptoms were identical to those observed in long-covid. The research of Dr. Bruce Patterson shows that viral infection can cause inflammation of the vasculature. This compromises contractions of the blood vessels needed to return blood to the brain from the lower extremities. This is usually described as ‘brain fog’ from hypovolemia. Three years ago I began taking ivermectin(IVM) and I was pleasantly surprised that it in a very short time it reduced my POTS symptoms by about 90%. I rarely have any symptoms of POTS now and I think it would be worthwhile to test IVM as a novel treatment. But, I’m not holding my breath since ivermectin was sorely maligned to encourage people to use the experimental gene therapy injections.