

Mestinon moves the needle on ME/CFS in unusual one-shot exercise study

The goal was to demonstrate in a one-day test that Mestinon has the ability to improve blood flows and cardiac output in ME/CFS and pave the way for a bigger clinical trial
This is the most unusual clinical trial/study that I’ve come across. Too short to be a full-blown clinical trial, it was essentially a short-term test of the idea that problems with neurovascular regulation (i.e. blood vessel flows) are inhibiting energy production during exercise In ME/CFS and – drum roll … can be improved by a drug.
It tried to do two things: it tried to show that neurovascular problems were affecting energy production in ME/CFS and that a drug could help ameliorate those problems, increase blood flows to the heart, and improve one’s ability to exercise.
In a sense, it was an impossible task. Since the study, “Neurovascular Dysregulation and Acute Exercise Intolerance in ME/CFS: A Randomized, Placebo-Controlled Trial of Pyridostigmine“, took place over one afternoon, there was no time for the full strength of the drug to show itself. Still, the authors hoped that a one-time shot of the drug would move the needle on blood flows and energy production – and thus lay the groundwork for a larger trial.
It did require finding 50 people willing to experience the joy of having a catheter inserted into their arteries and veins – engage in exhaustive exercise – rest for 50 minutes (catheters still in place, I imagine) – and repeat the exercise test! What a fun afternoon that sounds like. So a big shout out to the 50 people willing to go through some pain to support this study and this field. A big shout out to the Open Medicine Foundation Eliassen Fund that funded this study as well.
The authors explained two ways – both of which could be present – that may be impairing blood flows (e.g. hemodynamics) in ME/CFS. (Other ways of impairing blood flows may be present but were not germane to this paper.)
(1) Damage to the small nerve fibers (small nerve fiber polyneuropathy (SFPN)) found on the smooth muscles lining the blood vessels was one. (The smooth muscle cells lining the blood vessels are responsible for constricting or dilating the blood vessels).
It’s an intriguing idea. Skin and eye studies have generally found reduced numbers of small nerve fibers as well as narrowed small nerve fibers in from 30-50% of people with fibromyalgia, ME/CFS, and/or long COVID.
Because the small nerves could affect so many organs and parts of the body, a small fiber polyneuropathy (SFPN) that interfered with blood flows could conceivably explain much of what is going on with these diseases. We don’t know what’s causing the SFPN, but an autoimmune process would certainly fit the bill – and so could, apparently, in what was a rather shocking recent discovery – natural killer cells.
The role these damaged nerves may play, however, is not clear yet. An earlier study did not find that the amount of small nerve fiber damage found was associated with reduced blood flows during exercise; i.e. more small nerve fiber damage was not associated with worsened blood flows during exercise. That study, though, was not able to assess the deeper small nerve fibers that are more likely to play a role in this process. (A study to assess that is reportedly underway).
(2) Damage to the nerves found in the nerve ganglion (ganglionopathy) that impairs the release of two neurotransmitters acetylcholine and norepinephrine.
Pyridostigmine bromide, or Mestinon, potentially fits the bill for ME/CFS because it may be able to overcome the effects of a ganglionopathy by boosting the availability of acetylcholine and norepinephrine. Mestinon is a “reversible acetylcholinesterase inhibitor”; i.e. it blocks the acetylcholinesterase enzyme from breaking down acetylcholine in the synapse between the nerves. That immediately increases acetylcholine levels and norepinephrine levels further down the line.
Acetylcholine is the main neurotransmitter of the parasympathetic, or cholinergic, nervous system (PNS), or the “rest and digest” system. Studies suggest that the PNS – which regulates the sympathetic nervous system (or fight/flight) system – is impaired in ME/CFS.
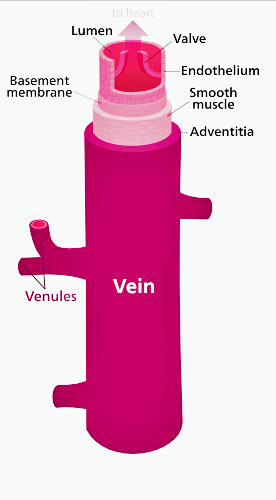
Note the smooth muscles lining the veins. Mestinon’s job was to help them tighten up the veins and send more blood back up to the heart – thus improving cardiac output and energy production. (Image by Kelvinsong,-CC-BY-SA-3; Wikimedia Commons)
By increasing acetylcholine levels at the nerve synapses in myasthenia gravis, Mestinon enhances the signal that tells a muscle to contract – thus reducing the muscle weakness found in that disease. Muscle weakness is not the primary problem in ME/CFS, though, and it wasn’t the acetylcholine angle that these researchers were interested in. It was norepinephrine (NE).
NE is a sympathetic nervous system neurotransmitter that constricts the blood vessels, and by doing so, potentially increases blood flows to the heart (improving preload), cardiac output, and aerobic capacity, particularly when standing. (All three of those are reduced in at least a subset of people with ME/CFS). Properly constricted blood vessels are needed to produce the pressure (vascular tone) needed to force blood back up to the heart and out to the muscles during standing and upright exercise.
Mestinon is also particularly effective at enhancing blood flows to the slow-twitch muscles that we rely on for activities requiring endurance and which use aerobic energy production to power them. A recent long-COVID study and an earlier ME/CFS study suggested that a wholesale conversion of slow-twitch to fast-twitch muscles may be occurring in these diseases.
Marrying POTS and ME/CFS
In a recent interview with the Open Medicine Foundation, Systrom said he was, with his colleague Peter Novak, trying to “marry” the results between the different stress tests done in ME/CFS and POTS; i.e. between his invasive exercise studies in ME/CFS and the tilt table studies done in POTS. Indeed, Mestinon popped up in ME/CFS largely because of its prior use in POTS.
The Gist
- David Systrom’s Mestinon trial in ME/CFS was an unusual one. It was a one-shot, one-day duo invasive exercise trial that tracked the ability of Mestinon to attack one of the exercise-related problems Systrom has found: the inability of the veins in a substantial subset of ME/CFS patients to firm up enough to send normal amounts of blood back to the heart.
- Those low blood flows back to the heart (low preload) result in reduced cardiac output and ultimately less blood flows to the muscles and less energy production.
- This problem, which is also found in postural orthostatic tachycardia syndrome (POTS), is apparently caused by nerve damage to the smooth muscles lining the blood vessels which inhibits the veins from contracting enough when we stand and during exercise.
- Mestinon blocks the activity of an enzyme that breaks down acetylcholine in the nerve synapses. By doing so, it increases both acetylcholine and norepinephrine levels. It’s the norepinephrine’s ability to tighten up the blood vessels that Systrom and his team were after.
- After the 50 patients in the study did an invasive exercise test, half were given Mestinon and half a placebo. They rested for 50 minutes and then redid the exercise test.
- The patients who were not given the drug exhibited an increased amount of energy production during the resting period – suggesting that their sympathetic nervous systems remained active. During the second test, their ability to produce peak amounts of energy dropped.
- Mestinon reversed both these trends. It stopped the energy drain during the post-exercise period – allowing their systems to rest – and improved their ability to produce energy during the second test. Mestinon did not improve oxygen extraction or the ventilatory inefficiency (hyperventilation) present in the group.
- Clinically (eg symptomatically) the changes were not significant and fatigue was not helped. Mestinon may not, however, have been expected to produce significant clinical changes in this one-shot trial, as its effects usually show up over weeks to months.
- With evidence in hand that Mestinon has the ability to move the needle on ME/CFS patients biologically, the authors called for more extensive and longer-term Mestinon trials in ME/CFS to be funded.
- With several processes (lax veins, mitochondrial problems, left-right shunt of blood away from the muscles, hyperventilation) potentially affecting energy production in ME/CFS, Mestinon provides the opportunity to improve one of them (lax veins that prevent proper blood returns to the heart). Systrom is in the midst of a clinical trial of a mitochondrial enhancer that may affect another.
When those POTS patients stand, their veins fail to constrict enough to overcome the gravitational flows of blood downward. This causes blood to pool in the lower body, thus reducing blood flows to the brain, and causing symptoms very similar to those seen in ME/CFS. These blood vessel failures appear to be the result of nerve damage in the lower bodies of POTS patients. This blood pooling is why compression stockings can be helpful in POTS.
A 2005 Vanderbilt study found that Mestinon (30 mg) significantly reduced the standing heart rates and symptom burden of POTS patients 2 and 4 hours later. The authors speculated that Mestinon helped the parasympathetic nervous system rein in the increased heart rates, while improved sympathetic nervous system functioning helped improve the blood flows in the lower body.
Systrom’s finding that only upright exercise tests reveal the problems present in ME/CFS suggests that something similar may be happening in this disease.
Study Goal
The goal of this study was to determine if a drug designed to improve nerve functioning and increase blood flows would work in ME/CFS. If the drug improved matters during an exercise test, it would show that problems with the nerve-blood vessel interface that’s responsible for moving blood back up to the heart is contributing to the exercise intolerance in ME/CFS. Plus, it provided data on the effects of exercise on blood flows, cardiac output, oxygen use by the muscles, etc.
This was also the first duo invasive exercise study ever done in ME/CFS that I’m aware of. When 2-day non-invasive exercise (CPET) studies are done, they’re usually done a day apart. These invasive CPET tests were done, though, 50 minutes apart – potentially giving us an interesting assessment of what happens very quickly to people with ME/CFS after exercise.
Results
Effects of Exercise
The study found that exercise caused the systems of the ME/CFS participants who had received the placebo to remain activated after exercise – causing a further energy drain. This showed up in increased resting oxygen consumption (energy usage) and cardiac output during the 50-minute rest period before the second exercise bout. Peak VO2 was also decreased in the patients given the placebo.
The authors proposed that an inflammatory response triggered by the exercise had, among other things, jacked up the production of reactive oxygen and nitrogen species (free radicals). They also suggested, as past studies have shown, that exercise produces prolonged activation of the fight/flight or sympathetic nervous system.
Effects of the Drug
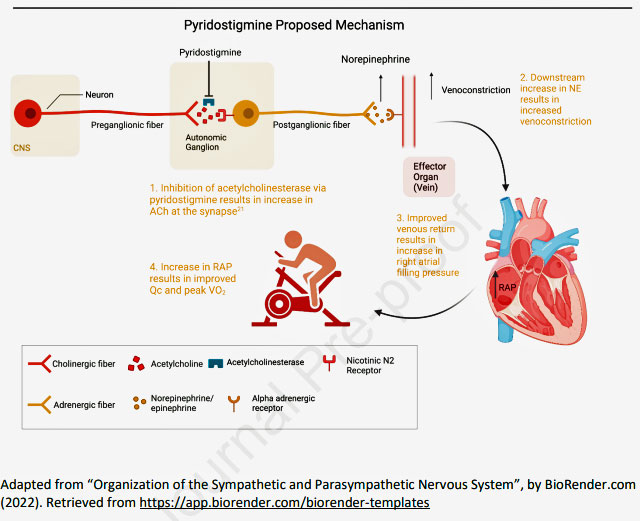
Mestinon’s proposed mechanism (taken from the paper). Notice how Mestinon affects “post-ganglionic” nerve transmission; i.e. it attempts to compensate for low levels of acetylcholine due to nerve damage by stopping an enzyme from degrading acetylcholine in the nerve synapse.
A key finding was that peak VO2 – the maximum amount of energy produced at a point during the exercise test – decreased in the patients given the placebo but increased in the patients given Mestinon. With this finding, two hypotheses were confirmed – problems with blood flow do contribute to the energy problems during exercise in ME/CFS – and they can be ameliorated at least somewhat with Mestinon.
How much, we don’t know. The “effect size” of this single-dose experiment was small; that is, while Mestinon did improve the ability to generate peak amounts of exercise, the effects were not “clinically significant”; i.e. it did not improve fatigue. Smaller amounts of Mestinon were able to quickly improve both fatigue and reduce standing heart rates in POTS patients, but Mestinon was given a much more difficult task in this trial.
Instead of testing Mestinon’s ability to improve standing for ten minutes, this study asked the drug to improve the ability to exercise in a second maximal exercise test taken 50 minutes after a first exercise stressor – a far more demanding task. Indeed, Systrom noted that the exercise test appears to be picking up more problems with preload and dysautonomia than the tilt table test.

Mestinon appeared to stop the strange post-exercise energy drain found, allowing the ME/CFS patients to return to a normal resting state more quickly.
Mestinon also stopped the after-exercise energy drain; i.e. the increased oxygen consumption (peak resting VO2) that showed up during the resting period in the patients who didn’t get the drug. In fact, instead of increasing their oxygen consumption, the people receiving Mestinon actually significantly decreased their peak oxygen consumption during the rest period. Similarly, the decreased resting right arterial pressure (RAP) in these patients also suggested that Mestinon helped the systems of people with ME/CFS return more quickly to normal after exercise.
Mestinon did not improve systemic oxygen extraction or energy production at the anaerobic threshold, or a number of other measures. This was probably anticipated, as Systrom noted in his recent talk that it often takes weeks or a month for Mestinon to take effect. Two conference reports indicated that long-term Mestinon use (> a year in some cases) in people with the kind of preload failure found in ME/CFS can indeed increase aerobic capacity. In those studies, it did not increase peak oxygen extraction or help with the problems with breathing found during exercise.
Breathing Issues
Possible breathing issues that occur during exercise have only recently received much attention in ME/CFS but seem to be gathering more attention. Systrom, for instance, has been mentioning hyperventilation for a while. Hyperventilation – also called over-breathing – refers to overly rapid or deep breathing.
This study found that a “ventilatory inefficiency” probably caused by hyperventilation (rather than “dead space” in the lungs) was present. Ventilatory inefficiency (VI) appears to occur, if I have it right, when our lungs move more air than is necessary to remove the carbon dioxide produced by the body when it produces energy aerobically. The “inefficiency” comes to play in high amounts of air moved relative to the body’s need to remove carbon dioxide. (It’s the need to remove the carbon dioxide produced during energy production that primarily triggers our need to breathe more deeply during exercise.)
Ventilatory inefficiency appears to be mostly found in chronic obstructive pulmonary disease (COPD) where it’s associated with dead space in the lungs, poor breathing regulation and early lactic acidosis, and is associated with difficulty exercising.
In ME/CFS, though, it appears to be associated with “poor breathing regulation”; i.e. hyperventilation during exercise, as well as early lactic acidosis and difficulty exercising. Hyperventilation has a disturbing knock-on effect: by reducing CO2 levels, it alkalinizes the blood, making it more difficult for red blood cells to lock onto oxygen molecules. Since it’s the red blood cells that carry oxygen to the mitochondria to be “burned”, this impacts energy production.
The hyperventilation may be another reason why Mestinon did not immediately improve shortness of breath scores, as it’s not clear that Mestinon has the ability to affect the breathing. (The authors noted, though, that this finding may be the result of a “false negative”, or Type II, statistical error that would be cleared up in larger studies.)
Mestinon has done very well for some people, but it was never expected to be a cure-all. As Systrom noted in his Open Medicine Foundation talk, multiple exercise issues have been found in ME/CFS. A drug that increases blood flows to the heart, and improves cardiac output by firming up the veins, may not be able to help with a right/left shunt in the peripheral blood vessels that drives blood away from the muscles, a mitochondrial problem, or hyperventilation.
Systrom reported that a mitochondrial problem could produce the same results in ME/CFS patients – and require a totally different treatment regimen. Calling the mitochondrial issue “relatively uncharted territory”, Systrom noted that some evidence suggests that viruses may be able to hack the mitochondrial genes (turning them off – a nice strategy if applied to the immune cells), or that infection-triggered oxidative stress could debilitate the mitochondria as well, and might even hit the small nerve fibers.
- Update – note that one small myasthenia gravis study suggested that Mestinon in combination with another drug might increase the risk of depression. One ME/CFS patient reported this happened with her.
Systrom – keeping his eye on every possible factor – is also in the midst of an $8 million 40-person ME/CFS mitochondrial trial funded by the Estrellas corporation. So far, 27 people have enrolled.
Systrom, who is now the co-director of the OMF’s Ronald G. Tompkins Harvard ME/CFS Collaboration saluted Ron Tompkins’ efforts to break down silos and build multidisciplinary teams to attack ME/CFS. That, he said, was Ron Tompkins’ vision, and that was how this disease is going to get defeated. Systrom also recently joined the OMF’s Scientific Advisory Board
Conclusion
The first exercise test resulted in increased energy consumption during the rest period, and reduced energy production (peak VO2, cardiac output) during the second exercise test. All the patients also demonstrated “ventilatory inefficiency”; i.e. a potentially harmful reduction in CO2 levels caused by hyperventilation.
The trial succeeded in demonstrating that blood vessel issues affecting the veins are impairing energy production in ME/CFS, and can be improved with a drug. While the effect size was not large (i.e. the improvements did not meet “clinical significance”), this unusual one-shot drug trial didn’t appear to be designed to do that.
Instead, the study showed that the drug has the potential to move the needle biologically in people with ME/CFS; it turned off the “energy drain” seen during the rest period, and improved peak energy production and cardiac output during the second test. Conference reports suggest that long-term use of Mestinon may be able to actually increase aerobic capacity in ME/CFS.
Mestinon was never expected to be a cure-all drug. It may not be able to affect other problems such as the shunting of blood away from the muscles, mitochondrial problems, and hyperventilation that appear to be spread across the ME/CFS population. It may be most helpful in a large subset of the ME/CFS population who have problems constricting the veins in their legs, and thus have reduced blood flows to the heart and the reduction in energy production that goes along with that.
We’ll see what happens from here. The authors called for larger, more extensive studies. Their demonstration that Mestinon can move the needle biologically in an area of great concern in ME/CFS seems to be exactly the kind of evidence that the National Institutes of Health purports to require to fund a clinical trial.
Biological evidence, after all, is apparently what carried the day for Shungu’s rare, successful application for an ME/CFS clinical trial. Dr. Rosa Maria Pari Ñaña’s recent Department of Defense grant to identify the subset of ME/CFS patients that are likely to benefit from Mestinon. Success could be very helpful in this regard.
If Systrom goes for an NIH grant, he’ll have to go outside the NIH’s ME/CFS program as it still does not allow for clinical trials. Still, he’ll have some good evidence to back him up.
Systrom alluded to the possibility of repurposing FDA-approved drugs for ME/CFS in his interview. In that vein, Mestinon would be a great “starter” drug for this disease. It’s not a mystery, it’s been around forever and doctors presumably would have no problem prescribing it. Having a drug like Mestinon associated with ME/CFS would certainly change the face of this disease, could help a large subset of ME/CFS patients, and prompt further investigations into one of the hottest areas of ME/CFS and long COVID – the blood vessels and energy production.






How was the Mestinon TOLERATED by the study participants?
I know it was a one-shot short test of brave volunteers (did anyone monitor how the two groups recovered afterward?), and no one is recommending the drug or effective dosing based on this, but how much it cost the volunteers, after a short study, is relevant – and not mentioned.
Not significant in any way? Random? Or simply not studied at all even with a follow-up questionnaire. Too late now, but I’d like to know if there was anything notable if it WAS done. Thanks.
That was not reported on. Mestinon has been used in POTS – a close cousin to ME/CFS – for quite a while and has been used by Systrom in quite a few ME/CFS patients. Aside from the hypersensitivity issue with ME/CFS – which can crop up any drug – I haven’t heard any particular concern about how well it is tolerated in ME/CFS vs other drugs (????)
Mestinon in all pill forms has either milk or corn allergens making it unusable for many ME/CFS patients. There is an oral liquid, containing uote a bit of alcohol for the recommended 10t daily dose, and a syrup with corn ingredients in it. It cannot be compound due to market manipulation of the raw ingredient.
Huperzine A, a cheap supplement, has the same mechanism, WITHOUT the allergens! Works well, with fewer side effects
The side effect I got on even a very small, subclinical starter dose of Mestinon was an increase in muscle aching to an unacceptable level. Painful aching muscles have been an ongoing symptom with ME/CFS for 27 years. I manage this by a lot of stretching, enough rest to avoid PEM, Gabapentin, and wearing only the softest, smoothest fabrics so as not to aggravate my SFN (actually polyneuropathy). I can keep in the narrow zone where I can pay attention to other parts of life instead of having it revolve around pain, but it is a close thing. So any drug which increases painful muscle contractions as it may be on its way to helping vascular laxity is unfortunately out of the question for a patient like me. Dr. Systrom mentioned this muscular pain problem almost under his breath at the end of an interview, saying it diminishes after months of use–but did not say at what dose or how long term or impaired these patients were to begin with.
I think Dr. Systrom and others should focus on the useful findings they are making about what specifically is awry in ME/CFS, and related conditions. This part of the research is great. Their experimentation with drugs may also be informative for research purposes, but to tout a drug as an effective treatment at this point in the process is premature. Maybe he has to pitch it this way to get research funding for ME/CFS patients, but it is putting the cart before the horse, to say it works before it has been properly tested.
I don’t think we-the-patients should be amplifying its as yet weak claim to be an effective treatment–implying that the side effects are acceptable too. A large detailed study over a possibly a year, using a wide range of patients whose ME/CFS symptoms run from “mild” to increasingly “severe”, and whose years of impairment also run the gamut–this would be settle the main point. However, I think what is actually happening is still more in the realm of basic research, and needs to be, before any claim that an effective treatment has been achieved.
Thanks, Learner – didn’t know about huperzine A – interesting! I’m going to give it a try.
Here’s an excellent resource on it – https://www.mghope.com/huperzine-a-mestinon-and-myasthenia-gravis/
Was the R Nase L test a scam cort? You said on Cheney’s thread Lombardi who owned Red Labs? said the test broke up the enzyme. Is this correct? If so, it must have been a scam like the XMRV test was which netted them around $2M. LOL
Please no one put the RNase L test in the “scam” box. It was developed by a small company, was used by ME/CFS experts for years, was believed by them and the company to be accurate. In fact, Kenny DeMeirlier wrote an entire book on it. It just turned out ultimately to be a mistake. I don’t think its been used for years.
I concur with learner. Huperzine A definitely works for some of us. Not to advertise, but dr. Diana Driscoll advocated this years ago. For myself it doubles the amount of steps I can do with an acceptable level of me/cfs related symptoms. Dosage is always the question…
What dose of huperzine A do you take, and what brand do you buy? How long did it take to notice an improvement?
I take Mestinon for POTS/fibromyalgia. So long as I don’t go over 15 mg twice per day I have no problems. If I do have too much the symptoms are really frightening, for me they are slurred speech, inability to swallow, drooling, muscle twitches and cramps all over, and shortness of breath. One time I took too much accidentally and could hardly breathe for hours it was hell. Covid test negative and it resolved as soon as the drug cleared. The study only involved small amounts of the drug and I’m sure they were fine because of that.
I found mestinon one of the hardest drugs to tolerate, I lasted 4 days of which the last 2 days felt like I’d been badly poisoned.
If I count start over I’d start with a dose so low my body wouldn’t notice it, and take weeks to build up tolerance.
Unfortunately after that poisoning sensation I tried that and I can’t even take a dose of Mestinon the size of a grain of sugar. I’ve tried building up slowly but as soon as I go over that amount I get incredibly unwell.
It’s like my body now remembers the initial full dose and reacts to any dose of Mestinon.
I only wish I started really low the very first time. And slowly built up over several weeks or months (like we do with LDN). Because now it feels like I threw away a promising opportunity from taking that first prescribed dosage.
What were your symptoms? I did have a severe reaction (cholinergic crisis I think) on the 30 mg dose initially prescribed and gave up on it. Then when I lost the ability to poop altogether last year I decided to try it again, at half the dose, and that worked. I can’t poop without it. It really helps me a lot for my POTS. I’m one of those people with poor venous return in my legs and SFN all over my body. You can always see the veins in my palms as they remain abnormally dilated. My feet turn blue just sitting on a chair for five minutes.
I do think you have the right idea to work up very gradually from the tiniest dose because it can help you a lot if you just don’t take too much of it.
I can only speak for myself but I’ve been on Mestinon since the study (Jan 2020) without any side effects. The jury’s still out on the. effectiveness – too many variable daily symptoms to measure well – but it may be helping and Dr Systrom has said it can take months to years to see the benefit, so I’m sticking with it.
Thanks Alicia for getting this thread going, and to Cort, Learner and Cecelia for adding crucial information!
ALWAYS my question – because, like many of us, I also always get the side effects, especially the bad ones. And I felt they were not mentioned. It would be sad if the only effective treatment investigated were one many of us couldn’t tolerate (the operation was a success – but the patient didn’t make it – comes to mind). Loyal opposition? Devil’s Advocate? Sigh. OTOH – if it works for some people, those people now have a treatment. And I AM happy for them.
I was part of the study and received the placebo. After the test, I had a full bag of saline via IV and I had no PEM at all.
Good to hear, Kelly! Thanks for letting us know. 🙂
So glad you had a safe result. Does the IV saline always work that way for you? I’ve never tried one; though I always ask for saline rather than a solution with sugar in it if I need fluids.
When I did the study, almost 3 yrs ago, I was not as sick as I am now. It was the only time I had the saline but my husband is looking into a home service so I can try it again.
I never started the mestinon after the iCPET – can’t remember why.
I had a crash, or am still in it, 5 weeks ago and now a simple shower causes PEM. I started mestinon about a week ago at 15mg. It didn’t prevent my latest PEM from the shower but I’m hoping it will help in time.
Trying the bathtub next!
A drug used against nerve agents like Sarin which has just been link with Gulf War Syndrome. Very interesting Cort
Sarin gas was recently linked with GWI – not pyridostigmine bromide. In fact, the sarin gas linkage was so strong that sarin gas is now thought to be the overwhelming cause of GWI.
https://www.healthrising.org/blog/2022/05/21/gulf-war-illness-breakthrough-chronic-fatigue-fibromyalgia-long-covid/
Besides, pyridostigmine bromide has a long history of effective use in POTS and orthostatic intolerance. A 2021 study found “clinical improvement” when it was used in POTS – https://pubmed.ncbi.nlm.nih.gov/29500811/ and a several hundred person study found it was effective in about 40% of POTS patients. A 2007 review concluded that it improved blood flows in POTS. https://pubmed.ncbi.nlm.nih.gov/17284509/
Other reviews routinely recommend it
It’s also as one review put it “been used for more than 40 years in the routine treatment of myasthenia gravis”.
It can clearly be helpful when used correctly.
I’d be impressed if they do a 2-day CPET test with it and show that the exercise ability stays intact on the 2nd day for CFS patients. Till then, it only proves that Mestinon is as effective as caffeine or Sudafed which we already know to temporarily boost exercise ability, but not exercise tolerance.
Oops, the above got under a reply by mistake…
A successful two-day exercise test would be the cat’s meow for sure but I don’t know of any treatment for ME/CFS that’s been tested that way. You might have to wait a while for that to happen. Hopefully it will.
Still, a long-term test of Mestinon is needed. As the blog notes some studies reported in conferences have found that long term use can increase aerobic capacity in ME/CFS. Given that I would give it the benefit of the doubt right now.
This study shows more, though, than Mestinon might be as useful as caffeine (which has not been tested in ME/CFS but who knows – might very well enhance ones ability to exercise in short bursts).
Mestinon was – aimed at a known problem – problems with blood flows – in ME/CFS – and was shown to enhance them and raise cardiac output and peak energy production. That finding in itself is pretty darn good.
I was in the Gulf War (and now have GWI/CFS, which fully manifested about 10 years after the war). We were given pyridostigmine bromide pills during the war (as a preventative measure, in case of a chemical attack).
I distinctly remember feeling very flu-like, and having bad headaches after a day of taking those pills. I was not alone, as many of my fellow soldiers who were also taking the pills said they were experiencing similar side effects.
We were not able to handle the symptoms, so we stopped taking the pills, and shortly after the side effects ended. In my option, the tolerability of this medication should be highly scrutinized, at the least.
To add, I am familiar with the recent sarin study. While sarin exposure has long been the leading theory as to the cause of GWI, I think it still has a ways to go before this is settled.
Yes, the study is very promising, but there are other causes/factors that have not been completely ruled out…potential biological weapon exposure, vaccine contamination/reactions, also there are service members that were not in proximity of the sarin plume on the ground, many who still reportedly have gotten sick (Navy personnel on ships in the Persian Gulf comes to mind).
I understand your experience, Jeremy but why do you believe that this – “the tolerability of this medication should be highly scrutinized” hasn’t already happened?
This drug has been in use for decades in myasthenia gravis, has been used a lot over the past decade or so in POTS, has been used by Dr. Systrom over at least the five years or so in probably hundreds of patients.
Wouldn’t you think they’ve been assessing the tolerability of this drug in their patients for quite a while. Why would you think this hasn’t been happening? To put another point on this – why would Dr. Systrom take the time and trouble to test a drug and do the whole invasive exercise study thing (a very expensive test to run) in people with ME/CFS – if he hadn’t already experience favorable results from it – and didn’t expect favorable results?
There’s also potentially a big difference between a healthy person taking a drug that boosts their normal levels of acetylcholine/norepinephrine to abnormally high levels and someone – like a POTS or ME/CFS patient who takes the drug to boost their low acetylcholine/norepinephrine levels to more normal levels.
Mestinon did wonders for me when my POTS was really bad, who knows, might have been responsible in part for my CFS “Remission”. Also helped loosen up my sinuses. Unfortunately it does make you bleed more, a challenge for us women, it can requires break every month. I had stopped it because I was so much better, but I seem to be in a crash so maybe I’ll try it again. Exercise tolerance has plummeted, and PEM has definitely become a problem again.
How come Myasthenia Gravis patients haven’t developed GWI after decades on this drug? Even for ME/CFS itself, we have several years of experience with Mestinon.
Dr Klimas was wasting everyone’s time. She got paid for years to solve the GWI issue, and somehow connected it to Mestinon. Now after years someone else finally connected the dots with Sarin.
From the sounds of it, the drug has potential and should be studied further. Just saying based on personal experience (and accounts of others) I have serious concerns about side effects …I don’t know anything about tolerability in studies/use with others but certainly hope it is minimal. Having CFS now, I would be willing try it again to see if it helped me…at the same time, I would be very nervous about having side effects again…but like anything I think there are always (potential) pros and (potential) cons that need to be considered. Even if they make a silver bullet pill that cures CFS one day, there’s bound to be a percentage of the population that will not be able to tolerate it.
Hi Cort. I did this study with Dr. Systrom in January 2020 at B&W Hospital in Boston. I may have received the Mestinon because my performance didn’t drop in the 2nd session on the bike. I’ve been on Mestinon since then with (maybe) some improvement…symptoms are always so variable that it’s hard to attribute the changes. It’s the same with LDN, which I’ve been taking since October 2021 – though the docs say both of these can take many months to kick in. Let’s hope Dr. Systrom’s results warrant more study. Thanks for the work that you do – it makes a difference to so many. .
I had an appt with Dr Systrom in March and he started me on Mestinon. What is LDN?
Low dose naltrexone – a compounded drug that can be very helpful – if you do a search on the website you’ll find many blogs on it. 🙂 Definitely worth a try.
Hi Cindi –
What Cort said… Dr Systrom didn’t prescribe the LDN. That was prescribed by Dr. Khosro Farhad (a neurologist who knows ME) at Coastal Neurology in Dover, NH. I’ve been to see him several times and found that he knows his stuff, listens well, gets to the point and I’ve left my appointments with him feeling like we’ve moved the needle (NPI). Good luck!
There seem to be some interesting treatment options emerging that are effective in at least some subsets of patients, but doctors treating CFS seem to do it more akin to doctors of the 19th century where their approaches are more of an art form than a hard science. That said, is there a listing of CFS doctors anywhere?
The MA CFS FM site – https://www.massmecfs.org/ – has a list of doctors by state. Good luck
The tools we have now are not very good at treating and diagnosing CFS. The FDA has not approved any drugs for CFS. We know little about CFS. Drugs is not only a therapeutic tool, but also a diagnostic tool. Only doctors who have a deep understanding of pharmacology and related physiology can have a personality to use medicine for everyone’s complex situation. That’s probably why you feel that way.
Hi Cort, A few years ago an article of yours prompted me to try Acetyl-L-Carnitine. I had some improvements from it. Another series of articles on Mestinon triggered some conversations with my cardiologist about Mestinon. He agreed to let me trail Mestinon because many of my symptoms were like someone with POTS. My heart preload improved. My standing heart rate decreased some. My sympathetic nervous system seemed to calm down. I was producing more energy, staying orthostatic longer, exercising more, and my life improved greatly. I’m not cured. I still have to watch my energy output. I still have to watch how long I am orthostatic. I still can suffer from PEM. I still suffer from many of the CFS symptoms. Bottom line, I am doing better because of Mestinon. Thank You Cort
Good to hear. This drug is not going to be for everyone and I don’t see it as a cure. I think of it more as a start – and as Systrom said – as a kind of “revealing” as in, if this drug helps – you probably have a problem with “loose” veins. Then the question becomes what exactly is going on with those veins and how we can better fix them. I imagine for many people other problems with energy production or blood flows may exist as well.
The big question is how to determine which subset of patients it can help with without having to go through a darn invasive exercise test.
So interesting that this helps some people. My son tried it two times and each time he declined in health. Disappointing and puzzling that it helps some and not others.
If I have it right – and I could be wrong – this drug will likely only help a particular subset of people – people with overly dilated veins. Note that some people believe the opposite problem is happening in some people with ME/CFS – overly constricted blood vessels
You can interrupt blood flows as easily by opening the blood vessels too large or by narrowing them too much. I imagine similar symptoms would result. As Systrom noted as well – a mitochondrial issue would require an entirely different plan of attack.
If your son is in the overly narrowed category I imagine a drug that further constricted the blood vessels would make things worse.
Thanks! That’s interesting!
I started out on 30mg 3 times a day and it helped, not a wonder drug but kept me ambulatory. As the dose increase I started having problems. I too am a Gulf War Veteran and I saw many get sick for not being able to tolerate the drug but after taking the drug for 3 years straight, it has only helped me. Do I have GWI symptoms? YES. But I had them before the Gulf War. Dr. Systrom, Dr. Amato, Dr. Doughty, Dr. Novak from Mass General Brigham are my treating physicians. Unfortunately, I have to many other comorbidities to be a candidate for many of the trials. I.E. In the CTE Study and BU, Empty Sella pituitary dysfunction and TBI with 4 fused disks in my neck just to mention a few. Bottom Line, Pyridostigmine does work for me even at a small dose.
Well – at least you are in good hands. What is I.E.?
Think ie means similar to eg or for example.
I am on Mestinon 60 mg BD. Maybe for 2 years now, I first read about Dr Stystrom and my New Zealand Rhuematologist was happy to let me try it. It has made a marked improvement to my exercise ability and recovery. Could not tolerate it three times a day. I have Fibro, fatigue and Auto immune.
It takes a while to get to this dose but for me it has been so worth it.
I can ride my horse 2-3 times most weeks, also a personal trainer x2 a week as able.
I have a history of CFS and polyneuropathy. After reading an earlier article on Mestinon, I asked my doctor (in the UK) to prescribe it. He wouldn’t but my neurologist had no objection. After much wrangling, I am now on my 4th day of 4 x 60mg a day.
My arms get fatigued easily and cause me pain, even typing this is problematical. I was hoping for relief from the lactic acid type symptoms but so far its been no help. Given its proposed action, I was expecting to find out if would help or not in a few days. I tolerate the tablet well and am instructed to take it 30 to 60 minutes before a meal.
I am unsure whether to continue.
Kevsy, I had to experiment with dosage before mestinon was helpful. It made an enormous difference, and your four tabs a day would have been WAY too much for me (at 230 pounds). Note that the tabs are scored for quarters, so it’s easy to try smaller doses. Eventually I found my optimal dosage could be met with half of an extended release tab (half of 180mg) per day (the ER tabs are scored in halves). I also discovered mestinon at night was unhelpful (since more tone in my muscles wasn’t useful for sleep), so in the evenings I transition to gabapentin, which I stopped taking first thing in the morning since it seemed to undermine the mestinon, and curiously, when on mestinon, I get no gabapentin withdrawal symptoms. So basically now I phase back into gaba in the late afternoon and rely on it for sleep. All of which is to suggest that getting the dosage right is essential.
Hey Paw,
Thanks very much for your reply. I have already seen that there’s no point taking it in the evenings. I did have weird cramps and fasciculations but I put that down to a v cold shower. I see the tablets are scored in 1/4s now. I’m at 170 pounds.
How long did it take to have effect? Do you get muscle pain/ nerve pain at all? Does Mestinon relieve any symptoms? 🙂
Kevsy, as I recall (from almost a year ago when I started) the worst symptoms from too much mestinon involved very bad cramping. But even at lower doses it slightly aggravated neurological symptoms (like burning) — but the trade-off was worth it. The main benefit being a distinct feeling of muscle tone that promoted exercise. E.g. I think I could literally feel tighter calf muscles with my hand. And the effects were pronounced with 20 minutes or so. After a couple of months the feelings were normalized but I’m pretty sure the effects were still helpful. In my mind I compared it to Adderall for the muscles, not the brain. I can’t tolerate Adderall regularly, but mestinon’s effects were limited to the extremities, in addition to having positive effects on digestion and IBS. (I’m speaking in the past tense because I haven’t been taking it for a few months; I had a long, bad flare-up in my feet from a complex of chronic tendon problems and gout, which I’m only now getting under control. I don’t believe the mestinon was related, as this is a long-term issue, but it certainly didn’t seem helpful to add muscle tone to the situation. I plan to resume the mestinon when all the swelling is gone.)
I was in touch a couple of years ago with someone who ended up doing very well on Mestinon but she, too, had to play around with the dose – until she found what worked for her.
Thanks Paw and Cort.
I was hoping that Mestinon would give me more stamina to avoid going into PEM.
I think my neuropathy pain has perhaps got a little worse on reflection. I’ll cut my dosage tomorrow. Take 30mg in morning then at lunch. And then rest in evening.
Best of luck Paw with your gout related symptoms. And thanks Cort for this amazing resource. I wouldn’t have tried Mestinon at all without your influence.
As someone who has been on Mestinon for about six years, you need to give it three -four weeks and get to the therapeutic dose.
Thanks, Mona for relaying that. It’s not unusual at all for it to take time to find the right dose and/or to allow the drug to take effect. It can take two months, for instance, for low dose naltrexone to take effect.
My daughter, age 19, is suffering from ME/CFS and POTS for over four years . A year ago she started with Mestinon, beginning with a low dose and is now taking Mestinon retard twice a day 1/2 a tablet with 90 mg.
She combines Mestinon with Propranolol 10 mg twice a day.
Before she started the medication, she spent almost all of her time at home, mostly in bed. On good days, she was able to take a small walk of 10-20 minutes, with rests in between in her wheelchair, but not every day.
On bad days, she wouldn’t leave her bed. She wasn’t able to concentrate for longer periods of time, she couldn’t go to school for a whole year, she wasn’t able to follow the lessons online.
After three month on Mestinon /Propranolol, she went back to school. Right now, one year later, she is in her final exams. Additional to her exams, she is able to meet with friends and can sometimes engage in “normal” activities.
Twice during the twelve month of medication she forgot to take her medicine. After a few days, she felt extremly exhausted and didn’t recover. Both times, she recovered after starting her medication.
Mestinon and Propranolol help her to have a significantly better life. It is not like a completly “normal”, healthy life, but the medication makes a huge difference.
She still suffers from postexertional malaise, but she recovers more quickly and the crashes are not as bad as they were before. I know that Mestinon is not helpful for everyone, but we are very grateful for a medication that improves her life in such a significant way.
It is also very nice to have an “old” drug that is helpful- well known towards side effects.
Cort, thank you very much for all the impressing work you are doing!
Silke
Silke, can you tell us what the Propranolol does for your daughter?
Are you allowed to name your doctor?
I already take a beta blocker. I have been diagnosed with lyme(4 yrs and still treating) and ME/CFS.
Thanks
I had a terrible experience with Mestinon, about 3 years ago, when Systrom’s research was getting more publicity. My doctor started me out on extremely small doses and on a very long introductory schedule, and I still had a terrible time. Felt worse than any crash I’ve ever had. It also took me several weeks to get back to “normal.”
I’ve heard somewhere (maybe on this site) that research is proceding on being able to determine whether patients — any patients — can tolerate a certain drug or not without actually having to go through a possibly negative experience. I hope there is some progress there.
As for the tolerability, my neurologist lifted his eyebrows when I asked about trying it, because of the lack of tolerability in his Myasthenia Gravis patients. His comment was that with Myasthenia Gravis there is almost nothing to lose, it can be so terrible a disease, so a difficult drug that’s only marginally tolerable makes sense. My CFS doctor at the time (well-respected) absolutely nixed it. But I wanted to try it and figured going very very slowly might make it work. It didn’t. Maybe Cort is right about the reasons why. I have no idea whether I have a loose or tight veins. I do have pretty severe OI as well as FM and a very sensitive gut so drugs are always iffy.
Tracey, I found a study from Moon et al: Efficacy of Propranolol, Bisoprolol, and Pyridostigmine for Postural Tachycardia Syndrome: a Randomized Clinical Trial, Neurotherapeutics 2018 Jul;15(§):785-795
I started research in the POTS field, because I couldn’t find much about ME/CFS. I’m a pharmacist, and I never was more happy about my profession. In this study they compared different groups: Propranolol, Bisoprolol, Propranolol+Pyridostigmine, Bisoprolol+Pyridostigmine. The physical components improved in every group after three month. The mental components improved only in the Propranolol+Pyridostigmine-group.
My daughter had a lot of problems with brainfog.
Propranolol is a very well researched drug, used for decades for millions of patients with cardiological problems and high blood pressure.
My daughter takes it in a very low dose, 10 mg twice a day. She normally has a very low blood pressure but the 10 mg have really no impact in lowering the blood pressure. Her heart rate in upright position isn’t rising as much as without Propranolol, so that is a very pleasant effect.
We didn’t experiment with a mono-therapy, because it works so well.
I know it doesn’t work for everyone. My wish is that more patients get the possibility to try it.
I’ve been on Mestinon for a while, for my POTS. It doesn’t seem to cause any bad sick effects for me. So, it appears that some of us can handle it.
If my supply gets disrupted, and I can’t take it for a while, my energy levels are most definitely lower.
Hey, Cort…I’m very interested in following Systrom’s ME/CFS mitochondrial trial funded by the Estrellas corporation. Do you have any further information or links you could share? Thanks for ALL you do to keep us informed.
Ehlers-Danlos people have high levels of fatigue and dysautonomia. We have a list of drugs to deal with these two symptoms and Mestinon is one of them. In a very informative book called ‘Disjointed’ the drugs are listed as follows;
“The list (for dysautonomia) includes five core medications which are most commonly used: fludrocortisone, midodrine, pyridostigmine (Mestinon), ivabradine and any one of a number of beta blockers (most often propranolol).
EDSers can have frequent pooling of blood in their lower extremities as well as tachycardia and other energy stealing symptoms. It is rather an art to apply these but many people find them helpful.
Now I would like to report on my own drug experiments; I have been gradually increasing my dosage of oxaloacetate to 800mg. per day and have not seen a noticeable increase in energy. Since it is so expensive I think I will drop it soon.
I’m having some success dealing with brain fog by using Vinpocetine and L-arginine to increase oxygenation, but I really think it’s the Vinpocetine doing the work– 30mg per day. Have also upped L-cysteine/NAC to 2,000mg. (glutahione precursor) as per my new wholistic M.D. at U.C.S.F.
This new doctor has also recommended that I go on a very restrictive diet; no dairy, no gluten, no lectins (a very lengthy list), and especially no soy, peanuts, lentils, kidney beans, white potatoes, or tomatoes. I hate the idea as I don’t have any gastrointestinal symptoms. I think I eat quite healthily–similar to a Mediterranean diet–but will give it a (short) try.
Also was at Stanford’s ME/CFS clinic recently and the new experiment is Ketotifen at night. Haven’t tried it yet. I’m very medication sensitive so am a bit worried, especially after reading comments from others who have tried this.
Dr. Bonilla chided me for my extra long supplement and medication list and said he gets everything he needs from the food he eats. I’ve had the thought that I should just stop everything for a while to just see…
He also told a long convoluted story about how just watching television was enough to put one of his patients into PEM, and how champion chess players could lose pounds of weight while engaged in competitions. I deduced that he was making a point that some activities which are perceived as innocuous in contributing to PEM, actually are overlooked.
So for the person who wanted to know if oxaloacetate helped, that is my report.
thanks, nancy, for your report. BTW, as a patient and as a med mal lawyer who takes the deposition of medical experts weekly, I have not met a mainstream MD ( especially those associated with a University) who don’t dismiss all vitamins and supplements out of hand. Western medicine is a form a religion where dogma seems to be handed down from on high ( i.e., FDA, big pharma, hospitals and medical universities.) I have read several books by MD’s who have been seriously ill, and when the system fails them, their eyes open to the outside possibilities. Comparing Huperzine to mestinon (above) is just another example.
I appreciate your report Nancy B. On what you are taking and what works for you, also from your new doc and the Stanford Center. Living in So. Cal, I am thinking at this point it is not worth going to Stanford. Since I am sure my ME/CFS Is at least partially virus related, it seems to be key to try to keep my immune system up (I wish there were something for us in that regard). I am going to try to go to see an infectious disease doctor who works in one of the long Covid treatment centers. Cort has said in a new funding blog some more or new funding will be coming to these centers and they will have to include ME/CFS people. Trying to get a head start there.
Anyone willing to do this is not sick. Imagine the people in “Unrest” being asked to do this. It’s a laugh.
And can we please stop coming up with ways to torture people and call it science? Who checks these things for ethics? It’s not worth knowing this. Come up with a better way to find this out, other than torture.
See Rachel’s comment, Stella
“I was one of the 50 participants! It was a rough day, but so worth it. I got answers to my mysterious health issues from the iCPET and contributed to ME/CFS research in the process.”
On the contrary, people willing to participate in these studies do it because they have hope that the outcome is going to be of benefit to them, and others much worse off, in the future. These studies are always subject to a panel of researchers, doctors and medical ethicists. From the two participants who were part of the study who have commented above it seems clear they were given ongoing access to the trial drug and continuing medical care. That alone is with it. We need more trials and more enthusiastic participation if we are to solve ME/CFS.
Cort- Thank you as always for the excellent update/summary!
And thank you for the shout out…I was one of the 50 participants! It was a rough day, but so worth it. I got answers to my mysterious health issues from the iCPET and contributed to ME/CFS research in the process.
I’ve been on Mestinon since my iCPET (August 2020.) I tolerate it just fine, but it hasn’t been a miracle drug for me. I still really struggle. But I think Mestinon makes my PEM a little less severe, so I’ve stuck with it.
Systrom switched me from short-acting 3x daily to long-acting 1x daily back in September 2021. I’ve been worse since. He’s *clinically* been seeing this and said he has some research into why. So he *advised me personally* to return back to short-acting 3x daily.
Good luck with returning to the short-acting form nd thanks for being in the study 🙂
My sympathetic GP prescribed thirty days of Mestinon for me because he had personally seen it work a medical miracle on someone essentially bedbound with a form of POTS. Unfortunately for me it made no difference, to include negative side effects. But of course you never know for whom it might be life changing.
The nausea and intestinal cramps were too much for me. I started on March 10 and it’s May 31, even 30 mg can start the nausea, etc. exploring other options.
Peter is that in the UK? Which city? My GP doesn’t prescribe anything since I moved to coventry. It sucks here.
I now think blood flow issues, are part of my collection of symptoms. Years ago, 2017/18, I was losing the strength to walk, any distance. My legs would just start to weaken. I was pale, thin, with lower blood pressure and heart rate. I was desperate to find help from something.
I remembered that I’d got a bit of energy from the Christmas chocolate and found some in the cupboard. I was able to walk further, without collapsing but I didn’t know why? I believe now, that the chocolate increased my blood flow because I’m intolerant to it. It puts my BP & HR up. I was delighted to find that I wasn’t completely broken. It’s taken me years since then, to try and find answers to other issues but it was like a jump start in my improvement. It would be interesting to see if Mestinon would have the same effect but as Learner says above, I have issues with corn, in particular.
We need nervous system drugs which target inflammation of the nervous and PNS not tests for ME and CFS. That can come later. ME is an enteroviral injury to the CNS and PNS. ENd of.
I’ve been taking 30 mg twice daily of Mestinon for years for myasthenia-like symptoms and it makes a big difference in my muscle strength and pain. I hesitate to call what I have myasthenia gravis because of Dr. Jaradeh’s single fiber EMG results. He said the results weren’t normal, but not typical for MG, and if the Mestinon makes a difference then I should take it. He apparently has seen other patients like me diagnosed with ME/CFS and Fibro who respond to Mestinon but don’t have the receptor antibodies or a significantly abnormal EMG.
My mom also had the same symptoms and test results and responded to Mestinon as well. Perhaps there’s a genetic cause for this, like faulty acetylcholine receptors, and it manifests as a myasthenia-like disorder.
I believe the brain is the ultimate
cause of cfs and pots. I believe the brain is signaling to the body that it is in consistent state of threat and therefore must conserve energy or “shut down” or protect the body leading to many symptoms and is why it often comes after stressors whether they are viruses (even when the virus is gone) or long periods of multiple forms of stress. This could also be attributed to all the other symptoms that pop up and Comorbid “syndromes.” In a nutshell I believe it is a maladaptive stress response. As someone who slowly began over years starting with just anxiety and watching the progression as I overtaxed my body, I have a different perspective than for someone who maybe developed problems over night by a virus.
Therefore, I think the focus should be studying the brain and what is going on there. There is a much better chance of figuring out ways to “cure” the entire issue that way.
However, delving in the bodily dysfunction is definitely better than nothings for symptom control and may assist in retraining the body and therefore the mind and systems as well. That said, I personally know many people who have healed from cfs and pots (not by pacing and even after being very I’ll for years ) through brain and nervous system plasticity training.
In regards to the physical issues, they are getting closer. I knew my blood was not pumping and circulating properly innately and also that ventilation is a problem. I believe there are multiple issues and the only real way to heal is to heal your brain and nervous system. Then the food allergies go away and sensitivities and allllll the other problems. Just my thoughts from experience and intuitive.
There was a German study in 2015 that showed elevated autoantibodies to M3 and M4 muscarinic acetlycholine receptors, as well as B2 adrenergic receptors. This was also confirmed by a Swedish study in 2020.
So by increasing the amount of acetylcholine available (as Mestinon does), essentially it has more chance of encountering and activating a ‘good’ muscarinic receptor amongst the ‘defective’ receptors. Perhaps this is a mechanism by which ‘loose’ vessels are tightened.
I’ve been using Huperzine A for some time for this very reason. Not certain how much improvement I see as on so many other supplements! I will mention Mestinon to my specialist as I also have MCAS and Acetylcholine acts on muscarinic receptors to inhibit the release of histamine from mast cells
Hey Cort,
Do you happen to know if Mestinon affects GABA? it doesn’t cross the BBB though, so maybe not.
I know that studies have shown that people with fibromyalgia later get dementia. I am thinking the mechanism here could be poor blood flow within the brain in these people. My mother, who always had pots and fibromyalgia symptoms, has frontotemporal dementia, and she is on donepezil which is I believe an acetylcholinesterase inhibitor, but different from Mestinon, in that it does cross the BBB. I wonder what I can do for the blood flow in my brain if I don’t have access to donepezil? I do exercise a lot and I find my cognitive function declines when I don’t. It’s just like when I go for a run it can help my ability to poop. Well, it also helps my brain work better and without that exercise it’s like I am in slow motion and I remember my mother was even worse, she moved like a sloth. It was the best she could do. But she was not an exercise lover like I am, and she paid a very heavy price for that. I am curious now, if huperzineA crosses into the brain. Before I got onto Mestinon, I took a supplement called 3Brains memory boost for many years, and it contains huperzineA. I’m just not sure that it does crossed into the brain however, I need to know more.
I’m in Australia and have ME/CFS and two types of dysautonomia (POTS and Neurocardiogenic syncope).
I’ve been on Mestinon 60mg BD for a few years now and it’s helped- no miracle cure, still have PEM and need to rest a lot etc, but it’s been noticeably helpful in reducing some intermittent muscle weakness I had especially in my legs, and has possibly reduced the severity of my PEM.
The only side effect I’ve had that I’m aware of is when I’ve taken the second dose of the day too soon (muscle twitching all over the body). Now I know how far apart to take my doses, that’s not a problem and I have no other apparent side effects.
One thing I was pleasantly surprised by was that Mestinon resolved my constipation (which started when my ME/CFS deteriorated and my POTS got worse). I know that some people experience stomach cramps and diarrhoea as a side effect of mestinon, but I have just had a resumption of normal gut motility which has been awesome 🙂
I would recommend giving mestinon a go, a couple weeks should be enough to tell if it’s helpful.
The biggest difficulty may be getting it prescribed. My ME/CFS- focused GP prescribes it for me, but regular GPs haven’t been keen.
Thanks for relaying your experience, R! Good luck with everything 🙂