
Pridgen’s effort to bring an antiviral drug combo to market is reaching its culmination. Meanwhile, he’s taking on long COVID.
Bringing a new drug combination to market is not easy. First, it requires creativity and initiative. Dr. Skip Pridgen was nothing if not creative when he put together a two-drug combo antiviral (valacyclovir) and an anti-inflammatory with antiviral properties (celecoxib) to fight fibromyalgia – a disease that virtually no one had connected to herpes viruses.
Not only that but he focused on a herpes virus – herpes simplex – that no one had thought seriously about in 30 years in either FM or its sister disease – chronic fatigue syndrome (ME/CFS). Plus, he employed a strategy that I had not seen before in ME/CFS – using two drugs to inhibit the virus at different points in its replication cycle to stop it from replicating. (The same general strategy is used to treat HIV.)
He showed initiative by raising $3 million for the first trial and enrolling top talent from Pfizer and other drug companies. He showed staying power by making it through the “Valley of Death” – the treacherous period between the stage 2 clinical trials and the stage 3 clinical where tens of millions of dollars need to be raised. (He expects the third and final stage of clinical trials for fibromyalgia to begin soon.)
Now he’s onto long COVID. As ever, he remains supremely confident in the ability of his drug combo to produce results.
Pridgen’s (First) Long-COVID Trial
Once again, he’s breaking new ground. While there’s been a lot of talk about herpesvirus reactivation in long COVID, this is the first clinical trial I’ve seen that is actually going after it.
Pridgen’s entry into the long-COVID field is really no surprise. He’s always believed herpesvirus reactivation plays a major role in the ME/CFS/FM spectrum of diseases. His provisional patent from over a decade ago proposed his protocol would work for a wide variety of poorly treated diseases including ME/CFS, irritable bowel syndrome, chronic pain, chronic headache, chronic neck pain, chronic back pain, chronic depression, chronic clinical anxiety disorder, post-traumatic stress disorder (PTSD), brain fog, cognitive dysfunction, and chronic interstitial cystitis. A published case report suggest his protocol might work for some migraine patients.
While Pridgen’s two-drug approach and his virus of choice are different, his general conception of the disease is not. He and his partner, virologist Carol Duffy, believe the bodies of people with fibromyalgia, ME/CFS, long COVID, and others, are overreacting to a virus that almost all of us carry.
“It’s basically exaggerating its reaction to the virus. Any little stress reactivates the virus, and, rather than the body saying, ‘Oh, this is just a virus I’ve been living with this since I was five,’ the body keeps saying, ‘Oh, my God,’ and throws on all this inflammation, and that gives these people this pain. There is a theory that all pain, one way or another, is inflammation. It’s inflammation gone awry.”
(That was ten years ago. Note that Pridgen has also found evidence of higher levels of herpesvirus reactivation in the stomachs of FM patients.)
He told me, “I am having excellent success treating long COVID and Cindy Bateman’s research (Bateman Horne Institute) has replicated my results.”
No papers have been published, but last month his company, Virios Therapeutics, reported the results of a pilot long-COVID trial done at the Bateman-Horne Center. Twenty-two female long-COVID patients getting the drug for 14 weeks were matched with 17 female patients being treated by either Dr. Bateman or another healthcare provider. The open-label trial meant that everyone knew what they were getting.
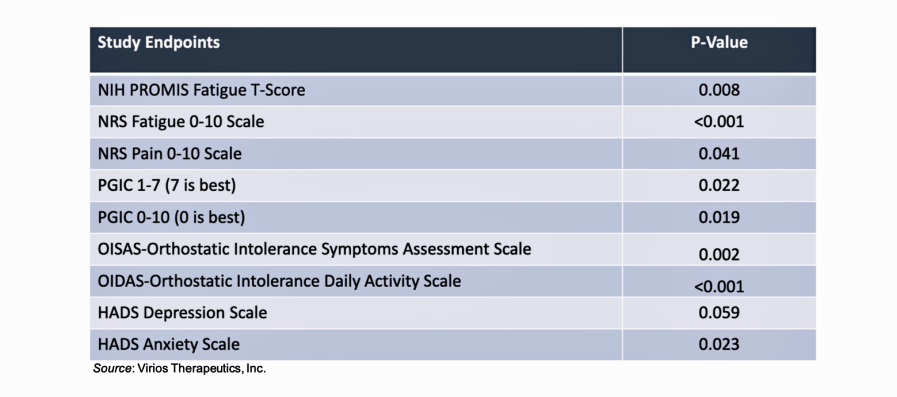
The patients getting the drug combo received “clinically and statistically significant improvements in fatigue, pain, and symptoms of autonomic dysfunction and general well-being related to Long-COVID”.
In ‘VIRI: Encouraging Results For IMC-2 In Long COVID…“, Zacks/SCR indicated that the improvements in fatigue were quite substantial. While the control group – which was being treated by other means – improved by only -0.34 on the PROMIS Fatigue score, the Pridgen protocol group improved by -7.24.
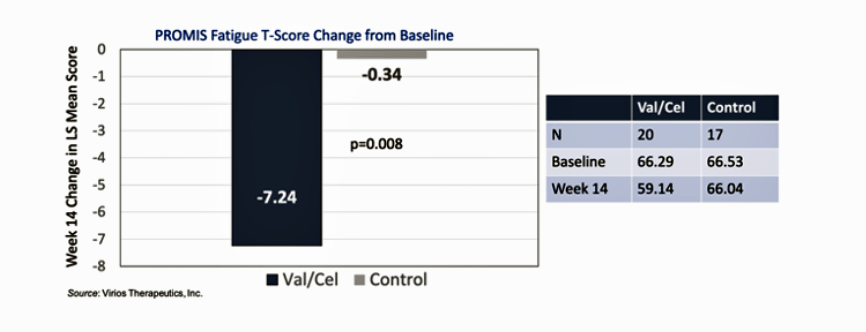
Note the dramatic differences in the fatigue scores between the antiviral and the non-antiviral treated groups.
If, as the report stated, a decrease of 3.0 or greater is considered enough for the patient to have experienced a “noticeable effect”, the effect these patients must have experienced was likely “very noticeable”. That reduction in fatigue suggests that a major impact on functionality – the most distressing symptom for many people with these diseases – may be possible.
Similarly, while the orthostatic (standing) symptoms of the usual treatment group appears to have actually gotten worse, the antiviral-treated group got better.
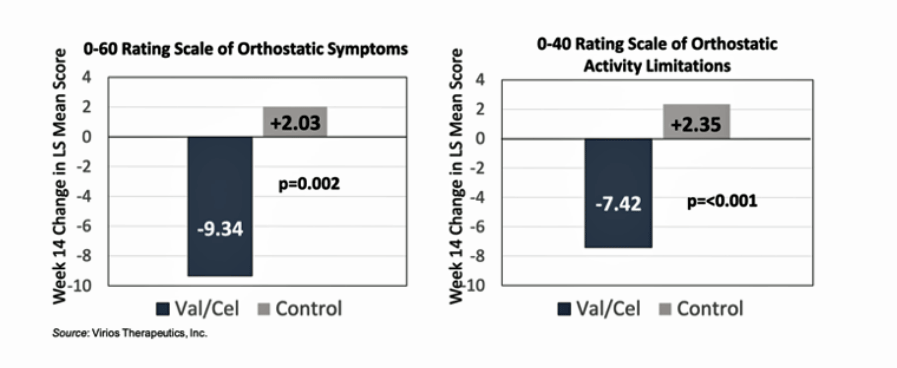
While the usual treatment-long COVID group appears to have gotten worse the antiviral treated-long COVID group appears to have received a substantial benefit.
Virios pointed to the wide range of benefits seen in the long-COVID patients.
THE GIST
- Dr. Pridgen’s attempt to bring a two-drug treatment regimen to market for fibromyalgia is into its 10th year, but he’s already moving into long COVID. This is no surprise, as ten years ago his provisional patent proposed the antiviral treatment regime would work for all sorts of illnesses including ME/CFS and irritable bowel syndrome.
- Pridgen’s assertion that reactivated herpesviruses – in particular, herpes simplex viruses – are causing fibromyalgia is notable given that herpesviruses have received almost no attention in FM over the past 30 years, and herpes simplex virus has been ignored in chronic fatigue syndrome (ME/CFS),
- Despite the many findings of herpesvirus reactivation in long COVID, Pridgen’s is the first effort that I know of to go after them.
- His company, Virios Therapeutics, recently released the results of a pilot study done at the Bateman Horne Center that included 22 long-COVID patients treated with the antivirals and 17 long-COVID patients who got regular care for 14 weeks. It was an open-label study – meaning that everyone knew what they were getting.
- The results were surprisingly good given the small size of the study. The patients getting the drug combo received “clinically and statistically significant improvements in fatigue, pain, and symptoms of autonomic dysfunction and general well-being related to Long-COVID”. The improvements in fatigue were particularly notable.
- Virios Therapeutics was clearly happy with the results and called the wide range of outcomes which reached statistical significance “highly encouraging”. Given the exploratory nature of the trial and the fact that it was “not powered to show significance” (which it clearly did), the company called the results “all the more impressive”.
- The path forward for fibromyalgia is a bit murkier. When one cohort of an FM phase II study received excellent results, another one did not. A deep dig into the stats revealed that FM patients who had been in past clinical trials (“experienced”) did not do well in this one.
- That’s a pattern that’s apparently been seen before in depression and other illnesses. Henceforth, Virios will focus on FM patients new to clinical trials and use dedicated research centers to do its trials.
- While it’s unfortunate that all FM patients do not appear to benefit equally, Virios thankfully did not say that FM patients who had been sick longer or who had more comorbidities benefitted less. Dr. Pridgen reported that the big – and apparently determinative – Phase III trial in fibromyalgia is expected to begin shortly.
- Meanwhile, the results from a larger Tonix pharmaceuticals fibro-long COVID study are expected soon. Tonix is also in the last stages of its fibromyalgia trial.
The usual warnings apply – this was a very small study that was not placebo-controlled or double-blinded. We’ve seen good results in small studies tumble before as the studies scaled up. Pridgen and company have successfully shepherded their drug combo through thick and thin in FM so far – but it hasn’t been easy.
Murkier Path for Fibromyalgia
There was the less-than-scintillating first study which was hampered by the FDA’s restrictions on dosage. Then there was another trial where the primary endpoint – pain reduction – was met by one of the cohorts but not the other. Digging deeper into the stats revealed something interesting – FM patients who have been through clinical trials or were enrolled in a research database before did not receive statistically significant benefits, but FM patients new to clinical trials received both “clinically and statistically significant reductions in pain, fatigue, FM symptoms, and both anxiety and depression symptoms”.
Virios noted that this pattern has been seen before in clinical trials of depression and other illnesses where “experienced” patients do not do as well as “inexperienced patients”. Virios suggested that given the 8 treatment trials over the past decade, the FM community may be reaching a saturation point in the U.S. “whereby experienced trial subjects and those presently treated for FM may be characterized as becoming more refractory (less likely to respond) to therapy.”
They’ve also found that using study sites that do clinical trials as well as treat patients is not as effective as using “dedicated” research sites with more expertise that solely do clinical studies. They’re going to stick with dedicated research sites in the future and screen out patients with “a recent history of treatment failure or patients who have previously participated in FM clinical trials”.
One would hope that everyone would benefit from the drug combo, and his past studies have not had to exclude patients in the way the next one will, but in an industry where 90% of clinical trials for drugs fail, you do what you need to do to maximize your results.
Since the analysis did not mention duration or comorbidities – which presumably it looked at – hopefully being sick longer or having a more complicated disease picture is not necessarily an issue.
One also wonders about the effect that mood or depression may have had on treatment efficacy. There are Fred Friedberg’s ME/CFS “Uplift study” (blog coming up), many mind/body recovery stories, including Patrick’s recent one on Health Rising, the recovery stories Raelan Agle features on her YouTube site, and the dramatic physiological, psychological, and functional benefits that Donna Jackson Nakazawa – a person with a severe autoimmune disease – received from a year of mind-body work. (See Health Rising’s book series here) – all suggest that mind/body work might be a good adjunct to other treatments.
Tonix Long-COVID Trial Underway
Pridgen isn’t the only FM drug maker looking into long COVID. The results from Tonix’s larger (30-site!), 14-week, phase II study focused on fibro-long COVID are expected in shortly. Tonix believes approximately 40% of long-COVID patients meet the criteria for fibro-long COVID. Perhaps looking ahead to an ME/CFS trial, the company noted that the same symptoms in long COVID occur in ME/CFS as well.
“Common symptoms of Long COVID, including multi-site pain, fatigue, unrefreshing sleep, and cognitive dysfunction, or ‘brain fog,’ are also hallmarks of conditions like fibromyalgia and chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME),”
Its TNX-102 drug – which the company states reduces both pain and sleep – is in its final phase of study in fibromyalgia as well. Results are expected in the last quarter of this year.
Next Steps
With these results in hand, Pridgen reported that Virios has raised the funds for a Phase 2 double-blind placebo-controlled long-COVID study and is applying to the FDA to get it started. Because Pridgen and company have already done extensive toxicology studies, the process should be quicker for long COVID than for the fibromyalgia application – which is already in its 10th year.
With regard to fibromyalgia, Pridgen is simply waiting on FDA approval – which could come any day – to get the OK to do its big – and determinative – Phase 3 FM study.
Found this Blog Helpful?
Please Support Health Rising






Really n=22 open label, control 17 only females trial? No objective endpoints? Very impressive -:)
Pilot trial! While there are clear issues you have to start somewhere. It’s not like anyone in the ME/CFS or FM fields is rolling in money. (We are not cancer). The good news is that this small study sets Pridgen and company up for a bigger and better study.
It puzzles me that they didn’t attempt any objective measures – actimetry using smart watches would be easy to do surely?
That’s always puzzled me with regard to clinical trials in general. You hardly ever see that.
It wouldn’t matter if CFS got the same attention that cancer gets. Big Pharma doesn’t want a cure for CFS or Cancer. They don’t have one cure for one chronic illness. Just look at the lousy ineffective and dangerous cancer treatments out there. Do you really think that giving billions and billions of dollars to CFS would make a difference? I’ve been listening to Dr. Thomas Seyfried he has lots of video’s on Youtube. His treatment for cancer addresses starving the cancer cells with a ketogenic diet and a drug to reduce glutamine and he also says that parasite drugs like fenbendazole and Ivermection and others are good at killing cancer cells. Cancer cells can only use glucose and glutamine as fuel so a ketogenic diet will strengthen healthy cells and starve the cancer cells. It’s kind of interesting that the mechanism of parasite drugs to kill parasites is that they keep parasites from using glucose. I know I feel better when I’m on a ketogenic diet. A similar protocol is used to treat MS which is a ketogenic diet, pulsing parasite drugs and using Chlorine dioxide. Makes me wonder just how much parasites are involved with all chronic illnesses and if using them would help CFS.
oh waw, that is not good….all these tiny trials instead of working together globally and do good trials, also no open label or objective endpoints. good trials.thanks for clearing me up. could only read gist…can now ‘put it away’
You can look at this several ways – that it was just a small trial, etc. Big trials, though, require that smaller trials get done first. Pridgen started off with a small fibromyalgia and now he’s worked his way up to a very large FM trial.
The successful small, ;ong COVID clinical trial allowed him to get the funds to take the next step – doing a much larger, more rigorous long COVID trial.
With regard to ME/CFS we’re probably years aways from a trial. Let’s see how the FM and long COVID trials do. If they work out ME/CFS is obviously the next logical step and I assume Pridgen would be able to quickly find funding to launch those trials.
As to objective endpoints – very few FM or ME/CFS clinical trials rely on objective endpoints because we don’t have them. Until we can objectively assess fatigue or pain or post-exertional malaise we’re going to have to rely on symptom questionnaires.
thanks for explanation C=ort because I can only read gist or a short comment.
so desperate for ME/cfs , he does nothing. I forget so much, i have the feeling that whole the ME/cfs research is stuck or almost stuck. Much about long covid yes, but the rest???
I just talked with Theresa McDowell – an ME/CFS practitioner in Arizona – she’s really excited about the things that are happening. 🙂
thanks again Cort, wish things would happen here to…
Hi Cort, do you know if there is something for Belgium, bedbound to? for ME/cfs what she said is happening?thanks!
Really interesting research re: herpes simplex, which I’ve had my entire life. But no visible outbreaks (generally near my right eye) since being sick. Cort, do you know the dosage of valacyclovir used?
hi Cort,
i could only read the gist. saw ME/cfs there to. is Pridgen going to do trials for ME to? or has he done? thanks!
OK. So this must be a stupid question but if he is having success with using those two drugs together why aren’t other Drs using it? You’d think there would be anecdotal evidence by now that it’s working. For people that are extremely sick and willing to try it (I’m neither) you would think it would be worth at least trying. Personally, I’ll keep using anti-viral herbs for awhile longer in hopes of eventually finding something that will knock the little buggers out.
He does have a specific formulation – I don’t know what it is – but I think its manageable as, as I remember, he would gave it out to doctors when they asked – so some doctors have surely given it a try. I don’t know how well its worked or not. Pridgen always been very high on his protocol and he’s certainly had success at time and has had some some successful placebo-controlled trials. The phase III trial, of course is the make or break trial. It must be exciting to be involved…
Other doctors are using it. I’m at the Stanford Clinic and use Valtrex and Celebrex for years. I CANNOT be without the Valtrex. For me, it’s the difference between being bed-bound to being couch-bound with occasional forays into the outer world.
Thanks! Good to know it’s working for you!
Wow, that is amazing that it’s helped you that much. What’s the dosing? I haven’t been able to find a doctor yet familiar with the protocol.
I’m currently on 1000mg twice a day. When I was with Dr. Martin, it was more, but I can’t remember…definitely 3-4 times as much
Thanks so much for the reply! That is great to hear that the valtrex has been helping you.
Interesting! Is there a chance the people who’ve already been part of numerous studies (who didn’t improve ) could simply be way less susceptible to the placebo effect? Since it wasn’t a blind trial, the placebo effect has to play a major role, right?
The studies that didn’t do well in FM was a placebo-controlled study. You would think the placebo effect would be stronger in newer patients but placebo-controlled studies are designed to take care of that.
If there’s a strong placebo effect I would think you would see a stronger overall treatment effect (placebo effect + treatment effect) when the treatment is effective.
Likewise, if you have a smaller placebo effect the treatment should still be effective – just less so; smaller placebo effect vs treatment effect.
The key is that there’s a good gap between the placebo and treatment effect.
I was told by some CFS doctors that HHV antivirals (by themselves) help in the first 1-3 years of the illness, and then they don’t. Sounds like adding celecoxib does not make any difference.
I think Dr. Lerner would disagree. He’s gone now but we have several ME/CFS recovery stories from him using antivirals – usually for long periods of time, though. Dr. Dantini has also been treating FM with antivirals for years. Finally, Virios reported that past clinical trial failures affected the results- but didn’t mention duration – so hopefully that isn’t a factor. I’m sure those doctors had some reason for those statements but I wonder if they’re accurate.
We’ll really see about his novel treatment regimen with the big phase III study.
A bit disappointed to find out the fibro trial has yet to begin. I thought it was due for completion last year…but I suppose covid and the above reported issues with participants has delayed it.
I’ve got my fibro and CFS under control now but I get still get herpes reactivation constantly. Low doses of valacyclovir are no longer strong enough. I do contemplate taking Prigden’s existing research to my doctor to discuss whether I could try it, but I would prefer successful Phase 3 outcomes first.
Do we know what doses of the 2 medications are used in the trial protocol?
That may have been the phase II trial.
I don’t know the doses. I don’t know if they’ve been made public but your doctor may be able to get them from Pridgen if you/he/she are interested.
We’ll know about the Tonix results before Pridgen – should be available by the end of this year.
Cort, do you know of any other trials that just look at high doses of anti-virals?
My understanding of the Pridgen hypothesis is that the Celebrex has a different and complimentary impact on the virus replication. But I have a lot of hesitation about taking an NSAID long term due to their well established side effects. My stomach is very susceptible to issues and many things known to aggravate ulcers usually makes me feel nausea.
I wonder whether other agents such as methylene blue or botanicals would also help.
My stomach couldn’t handle the NSAID. I wonder if there’s a substitute. There was a really promising small antiviral study out of Stanford followed by a small placebo-controlled study that failed. there was also a methodologically flawed study from Martin Lerner that did well as I remember. Lerner used long treatment times to get his result.
Cort, do you understand what was methodologically wrong with Lerner’s study?
I consider myself one of the lucky ones when Dr. Pridgen first started treating me 5 years ago. His beginning trials were underway and he agreed to put me on the drug combination.
It took 7 months until I saw improvement (Dr. Pridgen has informed me it usually takes 5-6 months).
I have now been on the drug combination for 5 years and I have my life back.
`
Let me also add to any of those who doubt I had true ME, I can assure you I did. I first became ill at the beginning of 2013. Housebound pretty much, bed bound 90% of the time. Often spoon fed and unable to make it as far as the toilet. Excruciating pain and utter debilitating fatigue., Cognitive issues so bad I stuttered, shook and couldn’t tolerate even the vibration of someone walking past me. The list of symptoms goes on, too long for here.
`I lost my job, my life, my dignity and if I’d had a gun I’d not be typing this today. So believe me, I know the hell which is ME.
I was ill for 6 years and I tried EVERYTHING to get well. At one point I was driven (too ill to fly) laying flat for the journey `as I could not sit up, to a` German clinic specialising in ME treatment. I spent `almost 2 months there receiving 7+ hours a day of IV antibiotics and the likes. No improvement was gained.
It was ONLY Dr.Pridgen’s drug combination that got me to where I am today. I am recovered. I still take the drug combination and without any side effects.
I pretty much owe my life to that man, so if you read this Dr. Pridgen, thank you again from the bottom of my heart.
That is an amazing story Karin. Very encouraging to hear that the treatment worked for you and so nice to hear how successful it was for you, it gives us all hope.
Cort lets hope the next trial is a success and the treatment can be rolled out to others who desperately need it. I certainly have the Herpes Simplex 1 virus so it’s nice seeing that being mentioned in connection to illness as I have seen little about it before. Fingers crossed!!!
I was treated by Dr. Pridgen for almost 2 years with no success. I stopped because he had me taking all kinds of opioids for pain and it didn’t even help. He kept insisting I take it. He also insisted I get my gallbladder removed even though my GI Dr. did a gallbladder study and said it was normal and I did not need to have my gallbladder removed. I wasn’t willing to travel to Alabama to have him remove it.
I’m glad it has helped for some but not for me unfortunately ☹️
waw, so glad for you you have your life back! You write you “had” ME, thought (sorry brainissues are still on protocol?) but do you maybe know why Pridgen is (sorry as far as my brain gose) does not do a trial on ME/cfs?
Hi Konijn
It’s not for me to say exactly why Dr. Pridgen’s trial is mostly targeted at FM patients.
However, it’s my understanding that Dr. Pridgen views FM, ME & CFS under the same umbrella. It is the virus that is the target of the drugs, not the name of the illness you have been labelled with.
I was at my wits end when I came across Dr. Pridgen and to begin with, I had doubt, how could I not when I’d tried everything else available to me. But I never take a single day for granted now and I’ll be forever grateful that I did not give up. All of you out there, at that point, do not give up!
thank you so much! If i understand well, the FM trial from Dr Pridgen is in fact an FM-ME/cfs trial? or do I understand it wrong if you write he sees FM and ME/cfs under the same umbrella? Sadly enough I lay in Belgium, verry bedridden. I can shurely understand you never take a day for granted! No, we may not give up but here t is awfull. get and cbt or rot in hell, no interest of what is happening worldwide, etc I really hope that things from the US, Canada, etc will come our ways but have waited until ’94. And it is as if they do not look futher here, what happens in other countrys.
I am located in the UK Konijn so I know exactly what the attitude is here in Europe.
Dr.Pridgen was more than helpful in suggesting if I could find a doctor willing to work with him and follow his protocol then can advise how to treat. It took me 4 years until I finally found a doctor in the UK to listen and prescribe the drugs. Until then I paid for the drugs Dr.Pridgen prescribed myself.
He does believe that they are all part of the same spectrum. I think he started with FM because he mostly saw FM patients when he started this all off. Just chance I guess.
thanks Cort! fingers crossed!!!
Exactly Cort. He saw mostly FM patients but it is by no means a restricted treatment for FM.
Hi! Thank you for sharing your experience and it’s amazing that you recovered. Was this a full recovery though? Are you able to exercise without PEM?
I’m already on the valtrex for a few months now, I would not be opposed to adding the other antiviral if it meant no more PEM ….
Hi Jen.
Yes, full recovery. No PEM.
It is important that all three of the drug combination are used together. Valcyclovir, Celecoxib and Duloxetine (taken at night for sleep). I take only 20mg each night of Duloxetine that is the lowest dose possible. Do not underestimate the importance of finding a way to gain sleep. The Duloxetine helps with this.
Good luck and stick with it.
Great to hear, Karin. Can you share the dosages of Famvir and Celebrex that worked for you?
Hi Remy. Let me check first with Dr Pridgen to see if he’s OK with me sharing this particular info. I’ll get back to you but may take a few days.
In the mean time if you wish to look him up, he’s a surgeon in Tuscaloosa, Alabama. Google and you’ll find him.
Remy, I’ve checked with Dr. Pridgen and at this time has asked that I not share my particular dosage and that’s because ” each individual differs from individual”.
And this is exactly why I am suspicious of Pridgen. I hope you won’t take this the wrong way, because it’s nothing to do with you, particularly, but it’s unconscionable that Pridgen isn’t more forthcoming with this information on the doses he is testing in his patients when he knows full well that these drugs are available separately, cheaply and could help patients now. I hope you will reconsider your gratitude to him in light of his obvious prioritization of $$$ greed over the well being of those suffering. This has nothing to do with patient safety and everything to do with profits.
For those that are interested, other patients have shared their doses in the past and that information is available in my notes, but may be outdated at this point. I am happy to share.
Remy, I’d be curious. I have a great PCP who I think will be willing to let me try this combo. I’m going to discuss it w her. I know Dr Pridgen has offered to share his protocol with other docs in the past, so I may have my doc reach out to him.
I’m especially curious bc in the past it appeared he was using Famvir vs now valcyte?
So happy to read your goof news, thank you Karin.
Could you please tell me how to get in touch with Dr. Pridgen?
Many thanks
Inge
Dr. Pridgen is a general surgeon in Tuscaloosa, Alabama
Hello! I went to see Dr Pridgen years ago. I believe the combination of the two drugs did help but now I am in the worst pain ever. I am absolutely miserable and don’t know what to do to get some relief!
So sorry to hear Beth! The only thing I can think is to keep up with the news. Have you tried low dose naltrexone or cannabis products? Cannabis can sometimes just disappear my pain.
😊. I had the same thought!
Hi Tracey,
Tell me please if you succeeded in contacting dr Pridgen.
(I live in Milan, Italy)
Inge
Hi Inge, no I have not tried to contact Dr. Pridgen. I’ll talk to my rheumatologist at the end of this month, who usually has an idea about things that are working w people. Because of many more patients coming in suffering w long Covid, he has more awareness of protocols. It sounds like Dr. Pridgen is using a combination of Valcyclovir and the other med mentioned. I do get herpes and have taken plenty Valcyclovir, which has not helped me at all with ME/CFS.
Are you going to try to contact him?
Hi Tracey,
I would love to contact dr. Pridgen, but I have no idea how
to do so
So its alluded to in the article that there is a supposed over reaction of the immune system to the presence of a virus that has always been there. But its also alluded that it is the attempted activation of the virus. So what is the real damage..an autoimmune like response or the virus,? And if the virus how can it be thought of as an over reaction? I’m confused on the chicken vs egg..virus vs faulty immune.
Pridgen’s later results of the stomach biopsies suggests to me that the virus has broken through.
Very, very interesting. Thanks for covering that, Cort!
I could say so much since I respond very well to (Val-)acyclovir myself during inflammation episodes. At the same time I am actually okay with the fact that my doctors refused to treat me with acyclovir without the evidence of big studies. This forced me to learn to pace and manage my illness with other tools and luckily it is working. Acyclovir is a very strong drug and is liver toxic in the long run!
I’ll add just one thing for now: Clinically acyclovir is the drug to go to for herpes symplex type 1 and 2, but it has also a very good effectiveness for HHV6. I have seen in a medical school book that the activity (as infectiologists call it) is much lower in vitro than in vivo which makes infectiologists believe that it has no good effectiveness against HHV6. But probably it is not true.
I want to remind you of Bhupesh Prusty’s post-mortem study of brain tissue of ME/CFS patients. It’s HHV6 and EBV and not herpes simplex that they found!
My theory of the different results of the effectiveness of acyclovir in ME/CFS: There are probably different groups in ME/CFS. For example Prusty has found EBV and HHV6 in post-mortem brain tissue. As far as I know there is no antiviral against EBV. But acyclovir has proved good activity against HHV6 clinically.
Another point is that I suppose that acyclovir only helps with symptoms when you experience an inflammatory episode. But not when you don’t. The point is that you can be very sick with ME because you have mitochondrial and blood vessel damage and probably more from the inflammation episodes but you may not be in an acute phase of the illness (after a “crash”).
This differentiation seems very important to do when testing drug treatment. However, I’ ve never seen much discussion about these two different states in ME/CFS.
I suffer from fibromyalgia and would.live to be updated as to of and when a new remedy becomes available to try
I have Long Covid and have been on valacyclovir for recurrent Herpes Labialis infections…
I now have Gluteal Tendinopathy and a high grade tear in my Gluteus Medius. There is evidence that Valacyclovir can be a cause of Tendonitis.
This of course could be all coincidental but there is no other cause for the Tendinopathy, so I have now ceased all anti virals.
I started out with the late Dr. L. Martin Lerner in Michigan where I did 9 round of IV Cidofovir and then moved to oral antivirals. The Cidofovir completely removed EBV from my system…like my titres were zero and HHV-6 was significantly removed. I transitioned to Valtrex, orally.
About 4 months after recovering from Cidofovir, I was able to exercise, again…I built up to running over 5 miles non stop at a time, a couple times a week. I was able to maintain this for about 6 months.
Unfortunately, a devastating major life event plus overdoing it in the gym made me crash, and crash hard. This was in 2016 and I have not been able to achieve that level of recovery/ remission since.
However, I still am on oral Valtrex. For me, IT IS KEY. I cannot live without it. Otherwise, I am constantly cycling through reactivated EBV infections. For me, it’s the difference between bed-bound and feeling flu like to couch bound w/out flu-like symptoms.
Am I still fatigued? Yes. PEM? Of course. Brain fog? Yes. Do I feel achy like I’m coming down with something every evening? No.
Really interesting JBinAz – and thanks so much for sharing that. Were you able to retry the Cidofovir?
No, I have not been able to find any physician who is willing to do it. It is not without risk, but Dr. Lerner had major clout at the Beaumont Hospital system in MI. I wish he were still alive :'(
I’m curious how two generic drugs have enough of an upside financially to justify the huge cost of trials?
Thanks, Cort. For another excellent article.
Good question. They must somewhow as Pridgen has been able to raise a lot of money to test them. He got a patent on using them together and maybe that’s what he needed (?)