

Geoff’s Narrations
The Blog
The GIST
Wust’s team took a look under the hood at the muscles in deconditioned healthy individuals and those with ME/CFS and long COVID, and found some startling differences.
Rob Wust’s group has a penchant for doing interesting studies. In the latest preprint, “Skeletal muscle properties in long COVID and ME/CFS differ from those induced by bed rest“, the Wust group goes after the deconditioning hypothesis proposing that lack of activity is the main driver behind ME/CFS or long COVID symptoms. This has always been a difficult argument because deconditioning causes very similar symptoms to those seen in ME/CFS. Nevertheless, several abnormalities seen in ME/CFS are not seen in deconditioned people.
No one to my knowledge has done what Wust did when he compared the cardiovascular and muscle responses of deconditioned but healthy individuals (n=13) with those of people with ME/CFS (n=13) or l,ong COVID (n=13) after exercise.
This was a very difficult paper, and I hope I got it right (lol). You can get a shorter overview of the study in David Tuller’s talk with Rob Wust.
Results
“These results suggest that skeletal muscle oxidative impairments in long COVID and ME/CFS cannot solely be attributed to deconditioning.” Authors
We knew that the muscles of the healthy controls were going to take a hit when they became deconditioned. Deconditioning, after all, causes rapid reductions in muscle strength and the muscles to atrophy. The muscles also shift from running on fatty acids to running more on glucose, and the muscle fibers themselves transform from slow-twitch (type I, oxidative, fatigue-resistant) fibers to fast-twitch (type II, glycolytic, more fatigable) fibers, resulting in a reduction in aerobic energy production. Insulin resistance starts to show up.
Maximal aerobic energy capacity was, as expected, low in ME/CFS and long COVID and healthy controls. All the groups also displayed hyperventilation during the exercise test – an indication that they were breathing longer and deeper in an attempt to get more oxygen into their now weaker muscles. Even at lower exercise intensities, the patients however, displayed more breathing issues.
THE GIST
Wust’s team took a look under the hood at the muscles in deconditioned healthy people and people with ME/CFS and long COVID and found some startling differences.
“Patients are often told they’re just out of shape. Our results show that this is incorrect. The muscle changes in these patients are different from what we see in healthy people after prolonged inactivity.” Rob Wust
- Please note that this is a small study (26 ME/CFS or long-COVID patients; 13 healthy controls), and time will tell how its findings apply. The study showed, as expected, that some of the processes that occur during deconditioning are happening in ME/CFS and/or long COVID. In several instances (more hyperventilation during exercise; reduced muscle fiber cross-sectional area; reduced capillary density), the processes simply seem accelerated in these diseases.
- In other instances, though, something very different appears to be happening in the ME/CFS/long-COVID patients. Mitochondrial impairments may impair the normal extraction of oxygen. Plus, something called “E/L coupling efficiency”, which describes how efficiently the electron transport chain is functioning, was reduced in the ME/CFS/long-COVID patients, suggesting that proton leaks or other problems might be present.
- Significant reductions in type 1 fibers and increased levels of fatigue-prone type IIa/IIx fibers in the ME/CFS and long-COVID patients suggested that a pathological process is at work in them, one that increasingly relies on non-aerobic pathways to produce energy. Plus, reduced capillary density in the muscle fibers indicated the ME/CFS and long COVID patients’ muscles were not getting normal flows of blood/oxygen.
- The fact that no evidence of local hypoxia inside the skeletal muscle in patients with long COVID and ME/CFS was found, however, suggested that oxygen was not running out. The authors concluded that the exercise intolerance in ME/CFS symptoms is likely caused by mechanisms other than local oxygen deficits in the muscle or mitochondrial problems.
- With that – bang! – two major hypotheses seemingly flew out the door. The authors pointed to larger, more systemic problems, such as “stroke volume, peripheral oxygen extraction, and baroreflex sensitivity during exercise,” to focus on. Another AI Perplexity query proposed similar reasons, plus toxins (lactate, cytokines) that interfere with muscle contraction or “energy sensing”.
- It suggested that compensatory measures, such as increased blood vessel vasodilation to increase blood and oxygen flow through the remaining capillaries, could be applicable, Another possibility is that the muscles could, instead of stressing the aerobic energy system, very quickly start prioritizing glycolysis (anaerobic energy production) and stave off hypoxia in this way. It also proposed that toxins (lactate, cytokines) that interfere with muscle contraction or “energy sensing” could play a role.
- Could problems with sensing/regulating energy levels jive with Naviaux’s cell danger response hypothesis (cells hunkered down, refusing to respond), Hanson’s findings indicating that at a molecular level the bodies of ME/CFS patients are simply not responding to the signal to exercise, or abnormal calcium signaling (Wirth/Scheibenbogen hypothesis)?.
- mTORC1 problems may come into play, as well. The impaired mitophagy (mitochondrial autophagy) identified by Simmaron researchers could explain how PEM in ME/CFS occurs even in the presence of normal oxygen levels. They could also explain why a mitochondrial enhancer like Rapamycin might help in these diseases.
- If I got it right, these study’s findings were a headspinner. While the authors proposed examining the mitochondrial ultrastructure, their findings suggested that researchers should look to more systemic causes, such as assessing stroke volume, peripheral oxygen extraction, and baroreflex sensitivity (autonomic nervous system regulation) during exercise, to explain the muscle and other findings in ME/CFS.
Please Support Health Rising and Keep the Information Flowing
Mitochondrial Issues Present But Not Determinative
The mitochondria were working fine in the deconditioned healthy controls – not so in the ME/CFS and long COVID patients.
An anomaly emerged when the researchers investigated whether maximal oxygen intake was correlated with aerobic energy production and mitochondrial activity. If I have it right, this reflects the idea that even if enforced immobility had weakened the healthy controls energy production systems, the systems should still be working fine, albeit in a weakened or less productive state.
Indeed, when they normalized mitochondrial respiration (mitochondrial energy production) to the activity of a mitochondrial enzyme called succinate, the bed rest did not affect the healthy controls’ oxidative phosphorylation capacity; i.e., the healthy controls had fewer mitochondria to work with, but the mitochondria they did have were working fine.
Fewer mitochondria alone did not explain why ME/CFS and long-COVID patients were producing less energy, however. Instead, it appeared that impairments in the mitochondria themselves were holding back energy production. This suggested that some sort of mitochondrial defect (impaired complex I/II activity, proton leaks, or cristae structural defects) was present. The authors noted that bed rest usually results in fragmented mitochondria but not the structural changes (reduced cristae (folds)) that have been found in people with ME/CFS and long COVID. Score one for mitochondrial problems in these diseases.
An assessment of the relative contributions of the Complex I (NADH) and II (Succinate)-specific electron transport chain (ETC) pathways to total mitochondrial respiratory capacity revealed that healthy controls switched to a greater reliance on the NADH pathway to produce energy, while the ME/CFS/long-COVID patients did not.
Although the authors did not mention treatment, the metabolic shift (greater reliance on the NADH pathway) observed in the healthy controls suggests that a keto diet may be beneficial following bed rest. The fact that this metabolic shift did not occur in ME/CFS and long-COVID patients suggests that while keto diets may help, they may not reach core issues in ME/CFS/long COVID.

Mitochondrial problems were found but the authors did not believe they were sole cause of ME/CFS or long COVID.
Despite the altered mitochondrial findings and their assertion that more mitochondrial work needs to be done, the authors do not believe that mitochondrial dysfunction is the primary cause of ME/CFS. (“The lack of association of VO2 max with mitochondrial variables suggests that the reduced exercise capacity in ME/CFS/long COVID is not solely explained by mitochondrial respiration or enzymatic activity.”)
While mitochondrial problems exist, they believe that issues such as impaired oxygen delivery (blood vessel dysfunction, microclots, and the capillaries’ inability to transfer oxygen to the muscles) and/or reduced venous return may play a larger role in the exercise limitations observed. (Other possibilities include systemic inflammation that targets energy production, poor muscle activation, and impaired brainstem signaling.)
Recently, Wirth and Lohn proposed that the blood vessels that feed the blood vessels may be impaired in these diseases.
Different Cardiovascular Compensations
Differences were also seen in how the ME/CFS/long-COVID patients and healthy controls compensated for the reductions in energy production during the exercise test.
The bed rest group increased its baseline heart rate and then elevated it slightly during exercise to increase blood flow (oxygen) to the muscles. The ME/CFS/long-COVID group, on the other hand, dramatically increased its heart rate.
The response of the healthy controls was expected because deconditioning is typically associated with a higher resting heart rate and a somewhat elevated heart rate. This is because the cardiovascular system, although weakened, remains essentially intact, and only modest increases in heart rate are required to respond.
The big jump in heart rates, on the other hand, indicated that the cardiovascular system was not “intact” in the ME/CFS and long COVID patients. The authors suggested that the increased heart rates may be an attempt to compensate for either a reduction in the amount of blood pumped out of the heart (reduced stroke volume) or an inability to extract oxygen in the muscles.
Their findings appear to align with David Systrom’s findings in his invasive exercise studies. In many individuals, reduced blood flow to the heart (preload failure) results in a decrease in stroke volume, which, in turn, leads to a reduction in blood flow to the muscles. Systrom has called preload failure the elephant in the room and signature invasive exercise finding in ME/CFS. In some patients, the oxygen flowing to the muscles is not being utilized by them, either because of mitochondrial problems or because the blood is being shunted away before it can reach them.
Capillary Problems
Deconditioning ended up affecting capillary density differently in the healthy controls and the ME/CFS patients. As the healthy controls’ muscles atrophied, the number of their capillaries remained intact – resulting in increased capillary density.
Their ME/CFS patients’ muscles did not show signs of atrophy, but had fewer capillaries per muscle than the bedridden healthy controls; i.e., they were not able to provide the muscles with normal amounts of blood during exercise.
Normal oxygen flows in the muscles (!)
Muscle capillary density was reduced but evidence of low oxygen levels (hypoxia) was not found.
Reduced capillary levels would seem to set the muscles of ME/CFS and long-COVID patients up for a nice dose of hypoxia (low oxygen levels) during exercise. The muscles need more and more oxygen during exercise, but with fewer capillaries feeding them, they would gobble up as much oxygen as possible – leaving the blood depleted and in a state of hypoxia.
The authors assessed levels of myoglobin, the protein that transports oxygen to the muscle tissues, to assess the incidence of hypoxia.
If the muscles weren’t getting enough oxygen, they expected myoglobin levels to increase in an attempt to provide more oxygen. If the oxygen extraction by the mitochondria was a problem, meaning the muscles were loaded with oxygen that wasn’t getting used up, they expected myoglobin levels to decrease. (Note, though, that hypoxia can occur in certain conditions when myoglobin levels are not increased.)
I would have thought hypoxia would be a slam dunk. The low oxygen levels in the muscles would result in hypoxia, ischemia, increased free radical production, impaired mitochondrial activity, and facilitate a quick entry into anaerobic energy metabolism. Plus, increased lactate levels (a sign of anaerobic energy production) were found in the patient groups. It all seemed to make perfect sense to this layman.
Except it didn’t happen. Myoglobin levels were normal, and the authors concluded that the muscles of the ME/CFS/long-COVID patients were not experiencing hypoxia “likely because the ratio between local oxygen supply and utilization rates are maintained“.
This indicated that the blood flow in the muscles was adequate and they were not trying to extract more oxygen than was present; i.e., they were not screaming out for oxygen. It also suggested there is no intrinsic defect in the mitochondria that is strong enough to impede oxygen consumption.
This is where it gets really complicated!
With that – bang! – two major hypotheses seemingly flew out the door. Yet the low capillary density in the ME/CFS patients indicated that blood flows to the muscles are reduced, and the aerobic energy system has taken a hit. So, how to explain the fact that oxygen was not in short supply in the muscles during exercise?
The authors pointed to larger, more systemic problems, such as “stroke volume, peripheral oxygen extraction, and baroreflex sensitivity during exercise,” to focus on. An AI Perplexity query suggested something similar was going on: it cited dysregulated brainstem signaling that impairs autonomic nervous system functioning (baroreflex sensitivity), reduced peripheral extraction, and/or reduced cardiac output (stroke volume), all of which limit the total supply of oxygen.
(Note that Wust’s group recently found that intense exertion discombobulated the autonomic nervous system.)
But how could oxygen flow inside the muscle be normal during exercise when problems with oxygen extraction exist? Another query “Could problems with peripheral oxygen extraction occur even when the ratio between local oxygen supply and utilization rates are maintained in the muscles and myoglobin levels are normal?” to AI Perplexity – provided some possible answers.
The AI engine suggested that problems at the microstructural level could be preventing oxygen from reaching the cells. Shunting – proposed years ago by David Systrom – could also cause blood flows to miss the oxygen exchange sites on the muscles. Microstructural barriers caused by fibrosis (the development of fibrous connective tissue – could also make it difficult for oxygen to diffuse from the blood to the mitochondria.
Another AI Perplexity query asked “In what situations would low capillary-to-muscle fiber ratios and capillary density NOT result in muscle hypoxia when stressed by exercise?”. Apparently some forms of compensation can produce this situation.
Increased mitochondrial efficiency to overcome the reduced blood flows was one possibility that did not seem to apply (some studies do suggest, though, that the mitochondria are overactive), Increased blood vessel vasodilation to get more blood/oxygen through the few remaining capillaries appears, however, to fit well with Wirth/Scheibenbogen’s hypothesis.
The hypothesis states that the body is pumping as many (rather toxic) vasodilators into the blood as it can to open up overly narrowed blood vessels. Another possibility is that the muscles could, instead of stressing the aerobic energy system, very quickly start prioritizing glycolysis (anaerobic energy production) and stave off hypoxia in this way.
AI Perplexity also noted that toxins (lactate, cytokines) that interfere with muscle contraction or energy sensing could also play a role. Problems with “energy sensing” sounded to me like the system is failing, but the muscles don’t know it.
Could problems sensing/regulating energy levels jive with Naviaux’s cell danger response hypothesis (cells hunkered down, refusing to respond), Hanson’s findings indicating that at a molecular level the bodies of ME/CFS patients are simply not responding to the signal to exercise, and/or abnormal calcium signaling (Wirth/Scheibenbogen hypothesis)?.
mTORC1 problems may come into play, as well. The impaired mitophagy (mitochondrial autophagy) identified by Simmaron researchers could explain how PEM in ME/CFS occurs even in the presence of normal oxygen levels. It could also explain why a mitochondrial enhancer, such as rapamycin, might help in these diseases (while others don’t)
At that my brain was more than full, and I gave up trying to make sense of the normal oxygen flows in the muscles issue. 🙂
Muscle Fiber Shifts
Despite the fact that oxygen levels within the muscle did not seem to be affected, the muscles of the ME/CFS/long-COVID group did take a hit. Muscle atrophy – a common result of deconditioning – was expected in the healthy control bed rest group, and it was found. It wasn’t expected in ME/CFS patients, and it wasn’t found.
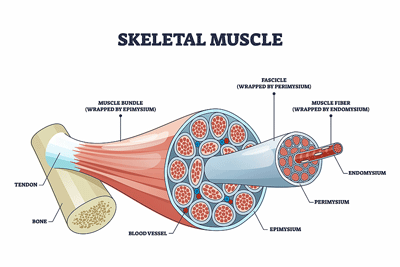
Decreased Type I and increased Type II muscle fibers in ME/CFS and long COVID suggested that a metabolic switch had been flipped.
The types of muscle fibers found in the patient and healthy control groups were, however, different. While the ratios of the types of muscle fibers remained unchanged in the healthy controls, the type 1 fibers in the ME/CFS and long-COVID patients, however, were significantly reduced, resulting in increased levels of type IIa/IIx fibers. These “fast-twitch” muscle fibers typically rely upon glucose for energy and quickly run out of energy during more endurance activity.
I asked AI Perplexity to determine if the muscle fiber shift found in the ME/CFS/long COVID patients was due to deconditioning i.e., “How to tell whether a slow-fast shift in muscle fibers is due to deconditioning or to something else?“. It responded:
“A slow-to-fast muscle fiber shift due to deconditioning is most likely when there is a clear history of inactivity, reversible changes, and no other pathological findings on biopsy. If there are additional clinical symptoms, family history, or pathological features on muscle biopsy, other causes such as neuromuscular disease, genetic myopathies, or systemic illness should be considered.”
While there is a clear history of inactivity in ME/CFS, since the inactivity is largely irreversible and other pathological changes have been found on biopsy, the bulk of the evidence suggests that the switch from slow to fast muscle fibers in ME/CFS and long COVID has a pathological origin. Several processes can trigger this muscle fiber switch, including mitochondrial dysfunction, metabolic signaling issues, and inflammation.
*Note that this research was partly patient-supported (ZonMw, Solve ME, ME Research UK, and the Patient-Led Research Collaborative).
Conclusion
“Patients are often told they’re just out of shape. Our results show that this is incorrect. The muscle changes in these patients are different from what we see in healthy people after prolonged inactivity.” Rob Wust
This was one of the most difficult papers to understand for me, and I am left wondering (more than ever) if I got it right. The study showed, as expected, that some of the processes that occur during deconditioning are happening in ME/CFS and/or long COVID. In several instances (more hyperventilation during exercise; reduced muscle fiber cross-sectional area; reduced capillary density), the processes simply seem accelerated in these diseases.
In other instances, though, something very different appears to be happening in the ME/CFS/long-COVID patients. Mitochondrial impairments may impair the normal extraction of oxygen. Plus, something called “E/L coupling efficiency”, which describes how efficiently the electron transport chain is functioning, was reduced in the ME/CFS/long-COVID patients, suggesting that proton leaks or other problems might be present.
Significant reductions in type 1 fibers and increased levels of fatigue-prone type IIa/IIx fibers in the ME/CFS and long-COVID patients suggested that a pathological process is at work in them, one that increasingly relies on non-aerobic pathways to produce energy. Plus, reduced capillary density in the muscle fibers indicated the patients’ muscles were not getting normal flows of blood/oxygen.
The fact that no evidence of local hypoxia inside the skeletal muscle in patients with long COVID and ME/CFS was found, however, suggested that oxygen was not running out. The authors concluded that the exercise intolerance in ME/CFS symptoms is likely caused by mechanisms other than local oxygen deficits in the muscle or mitochondrial problems.
With that – bang! – two major hypotheses seemingly flew out the door. The authors pointed to larger, more systemic problems such as “stroke volume, peripheral oxygen extraction, and baroreflex sensitivity during exercise” to focus on.

The authors proposed looking to systemic processes such as assessing stroke volume, peripheral oxygen extraction, and baroreflex sensitivity (autonomic nervous system regulation) during exercise, to explain the muscle and other findings in ME/CFS.
An AI Perplexity search suggested that compensatory measures, such as increased blood vessel vasodilation to increase blood and oxygen flow through the remaining capillaries, could be applicable, aka the Wirth/Scheibenbogen hypothesis. Another possibility is that the muscles could, instead of stressing the aerobic energy system, very quickly start prioritizing glycolysis (anaerobic energy production) and stave off hypoxia in this way.
AI Perplexity also proposed that toxins (lactate, cytokines) that interfere with muscle contraction or “energy sensing” could play a role.
Could problems with sensing/regulating energy levels jive with Naviaux’s cell danger response hypothesis (cells hunkered down, refusing to respond), Hanson’s findings indicating that at a molecular level the bodies of ME/CFS patients are simply not responding to the signal to exercise, or abnormal calcium signaling (Wirth/Scheibenbogen hypothesis)?.
mTORC1 problems may come into play, as well. The impaired mitophagy (mitochondrial autophagy) identified by Simmaron researchers could explain how PEM in ME/CFS occurs even in the presence of normal oxygen levels. It could also explain why a mitochondrial enhancer like Rapamycin might help in these diseases.
If I got it right, these study’s findings were a headspinner. While the authors proposed examining the mitochondrial ultrastructure in the muscles, their findings suggested that researchers should look to more systemic causes, such as assessing stroke volume, peripheral oxygen extraction, and baroreflex sensitivity (autonomic nervous system regulation) during exercise, to explain the muscle and other findings in ME/CFS.






This also makes my head spin Cort -:) Even though we do not yet know what the causes are for this mysterious exertion intolerance. It is good to see that there are now many exercise studies done by different groups of scientists around the world where these findings keep coming back (consistency). That is where science starts. What I find a pity is that no blood volume has been measured in all groups. Remarkable is the breathing (and low oxygen uptake). Most of us also experience a kind of air hunger, also at rest. Which gives an unpleasant feeling as if your body cannot relax. What I think is that ME patients are already in a kind of 1st or 2nd gear before they cycle. There is no rest point on a physical level as with healthy people. That is where the autonomic nervous system comes in as a suspect again and the brain stem.
Yep. It was a headspinner for me. I just gnawed and gnawed at the normal oxygen flows inside the muscle and problems with peripheral oxygen extraction being present paradox. Hopefully I was somewhat in the ballpark. These things are really complex.
Interesting that blood volume is not being measured – good point. Nobody thinks it’s the answer but it has to be part of the problem. It seems like another “sensing” problem: the blood volume is low but the system is not responding to increase it – like it doesn’t know it is there – or something else has gone wrong in the blood volume enhancement system.
“There is no rest point on a physical level as with healthy people. That is where the autonomic nervous system comes in as a suspect again and the brain stem.” I agree – and the ANS pops up again.
I’m sure Cort knows this about blood volume, but I just thought I’d drop it in.
Satish Raj and some other researchers found that in people with POTS who had low blood volume, the level seemed to have been reset lower than it ought to be, and whereas in a healthy person with low blood volume the body would be pumping out various signalling chemicals and taking measures to fix it, nothing was happening, as though the body didn’t realise anything was wrong.
The renin–angiotensin–aldosterone system (RAAS) takes a main role in regulating blood volume, but in those POTS patients it wasn’t responding as expected. Unfortunately nobody has been able to take the research further yet to see where the fault originated – was the RAAS not working, or was something else affecting the RAAS?
Here’s one of the articles:
https://www.ahajournals.org/doi/10.1161/01.cir.0000160356.97313.5d
There is a newly available, low-cost method to measure blood volume with an off-the-shelf device purchasable by hospitals and pathology labs that could make research in this area much easier:
https://detalo-health.com/
It would be nice to see this research repeated on a large group of CFS patients.
This is so interesting. I’ve always felt that my muscles (especially in my weak point, in my case my thighs) aren’t right somehow. I was told they were fine structurally (the muscle fibres), but just couldn’t get their energy up … it’s not scientific, but my internal perception of my muscles told me otherwise.
To me it feels like my legs are ‘rotting’.
Always thought that the ‘air hunger’ (good description!) was the muscles that pump the lungs being fatigued.
Great reading, Cort and thanks for your efforts to share these studies. I’m so happy that more research is confirming why exercise is so difficult for me patients and hopefully we’ll all learn what can be done.
This kind of research is really encouraging. Thank you for breaking it down for us Cort. Do you see it as a problem that the patient group was categorically mild? If severe patients could be studied I would have to assume the results would be more striking and we could then for sure cross the impaired oxygen and mitochondria theories off the list. Although I understand it’s a practicality thing.
Good point! The patient group had to be willing and able to withstand the rigors of an exercise test – a less affected group for sure.
What I keep thinking is that researchers should look for a control group of patients who do not fit the full set of ME/CFS criteria, but have pronounced PEM. So there’s more signal and less noise in data, i.e. less noise from the multitude of other dysregulations that increase with disease severity, and it might be easier to hone in on the root of PEM.
Wow, a headspinner indeed! I know this one was on the challenging side but you really do a great job as a science interpreter for this stuff. The fact is it’s mostly really complex material. But at least we know the simple part: we are NOT just out of shape!
Cort, how you get your head around these complex papers, especially when you are unwell yourself, blows my mind. I’m about a quarter of the way through this blog, and will have to take a break before coming back to it!
Coincidentally, I watched a “layman’s interview by David Tuller on YouTube yesterday about this paper:
Interview with Dr Rob Wüst about his new study of ME/CFS, Long Covid, and deconditioning
https://www.youtube.com/watch?v=a8RhrPKI9Pk
“layman’s”
Forgot to say, there are a couple of glitches in the video, where the picture freezes, and the connection is briefly lost.
Thanks, Fay – I included it in the blog 🙂
It’s interesting because my condition seemed to begin after a period of overexertion, and I’ve suffered from severe PEM. For a long time, I believed it must be purely physical—like I’d just pushed my body too far. But the more I learned, the more I realised something deeper was going on. The muscle changes, mitochondrial issues, and exaggerated heart rate responses all point to a nervous system that’s stuck in a dysfunctional state.
For me, that shift in understanding has been powerful. It explains why traditional rest, physical therapy, and especially graded exercise therapy (GET) didn’t help—and in fact, made things worse. Nervous system-focused approaches, on the other hand, have produced real improvements.
Also, in the video, Wüst says, “We only test people that have physical PEM.” That kind of defeats the object, because we know PEM can also be brought on by mental or emotional exertion. To me, that actually strengthens the argument that this is a system-wide dysfunction—not just a problem with the muscles, but with the nervous system’s ability to regulate energy, stress, and recovery across the board.
Cort, this is very interesting indeed.
To summarize Rob Wüsts work as presented here:
ME/CFS patients are found to have low capillary density in their muscles.
This indicates that blood flow to the muscles is reduced.
Yet, this does not seem to result in muscle hypoxia.
Also, mitochondrial function does not seem to be the bottleneck problem.
The problem (as many of us said again and again, me included 😉) seems to be a *central* one: muscle perfusion does not seem to increase in response to exercise as it should – due to dysregulated brainstem signaling.
In other words: instead of switching into the performance mode, the muscle stays in the chill mode.
And this is a problem.
Incidentally, this is exactly the same problem as seen with cognitive exercise (Rayhan/Baraniuk 2021)
The core problem of ME/CFS seems to be a central lack of adaptability to exercise (of all modalities).
Mechanically speaking: The connection between the accelerator and the engine is bust. Both may be fully functional – but they do not “communicate”.
For those interested in the evidence supporting this assumption: https://www.kinder-verstehen.de/wp-content/uploads/Renz_Polster_hypothesis_MECFS_122024.pdf
We´ll be getting there, sooner or later 😉
Great explanation, Herbert – thanks! Very helpful.
AI Perplexity and Wust both stated that problems with muscle contractions – because they’re not getting the signal from the brain or from ion channel problems – could be causing the muscles to “chill” :).
What a fascinating situation.
Thanks for the summary Cort!
This is why low-dose nicotine helps so many people. It has an effect on the brain/brainstem to reset the cholinergic system. While is also has effects on the cardiovascular system and at a cellular level. Almost every cell has nicotinic receptors.
Nicotinic receptors are the “messengers”. If they are blocked, dysfunctional, or insufficient, the whole system stops working. Our new paper from 2025 explains in detail how these interactions happen from many different angles. But it is not light reading either.
https://link.springer.com/epdf/10.1186/s42234-025-00167-8?
Hi Herbert,
Indeed, it is a fascinating topic. Would you consider the possibility of delayed or progressive sarcolemma damage, given that it is associated with a genetic marker?
I have several pathogenic “RS” variants linked to Dysferlinopathy, specifically affecting the DYSF gene, with symptoms beginning in early childhood (around age 5) and now showing progressive development. In addition to skeletal and limb-girdle muscular weakness, and currently residing in Germany, I contacted Prof. Spuler for further evaluation.
Prof. Spuler kindly welcomed me for testing; however, I unfortunately had to decline, as I currently live in Bavaria and the distance to Berlin is too great to manage at the moment.
https://www.mdc-berlin.de/news/press/developing-crispr-therapy-muscular-dystrophy
Best, Sieglinde
Hi Sieglinde,
I am very sorry, but this really not my area of expertise… Hope you find a specialist to help you on the way! Best, Herbert
This is so interesting. I’ve always felt that my muscles (especially in my weak point, in my case my thighs) aren’t right somehow. I was told they were fine structurally (the muscle fibres), but just couldn’t get their energy up … it’s not scientific, but my internal perception of my muscles told me otherwise.
Deep thigh pain was the first symptom I noticed….those big muscles…
Hi Roberta – I really appreciate your focus on thigh involvement somehow. Early on, I had deep anterior thigh pain, almost as if thighs were ‘filling up’ with the initial assault of early virus exposure, reactions to medications/ toxins/ vaccines and eventually with any overexertion. Muscles never seemed to develop there, like these fluid filled pockets were just taking up space. Then as I started guaifenesin without salicylate blockade, the palpable nodules or undulations of my anterior thigh, specifically the rectus femoris muscle, went down along with a lot of painful, nerve compressing nodules throughout the body. I have much less overall body pain but still no concrete explanation of why it works. Some think it has to do with phosphate/ ATP regulation which must improve mitochondria and calcium signaling, but it’s beyond me. All I know is I now have less body pain, less heart and spine pain (where these nodules were taking up space).
Interesting Denise … my go-to is a small grounding pad (just an inexpensive, strip style one from Amazon) laid across my bed where my thighs are. This addresses most of my thigh issues, except when I am extremely over-fatigued.
USA Dr. Paul Cheny, deceased, and Dr. Sarah Myhill, UK, both found 90-93% MECFS patients to have PFO, patent foramen ovale.
Interesting. I have very low blood pressure, so a vasodilator toxin would make sense. I also often feel like having lots of lactic acid in my body, but when finger prick testing, it is mostly normal. If I walk up the stairs in one go, I get what feels like capilary cramps / claudication pain, and I have to stop and wait for it to pass , and the same if I squat. Can’t do that. I feel like it is something wrong in my blood vessels.
While Cort is my “go to” for information, I also search the internet including Mayo Patient Connect and Reddit posts. I found this 2024 paper in one of these searches. It is 3925 patient reports of the treatments that helped them the most. The authors included Dr. Ron Davis.
https://www.researchgate.net/publication/386287078_Patient-Reported_Treatment_Outcomes_in_MECFS_and_Long_COVID
Patient-Reported Treatment Outcomes in ME/CFS and Long COVID
Cort, I apologize if you already covered this and I missed it.
Thank you for your post on Rob Wüst’s new study. I read a summary already a couple of days ago and I didn’t realise that it was also pointing to the fact that the mitochondria were not the problem.
What I don’t understand is why you and others here think that these finding are headspinning. There’s a research overview about the mitochondria by the British ME Association from 2019 that concludes that the mitochondria are not the problem but that research should look “upstream” – in the bloodstream – to find out what was in the blood that caused damage to the mitochondria and why it was there.
https://meassociation.org.uk/2019/07/mea-summary-review-the-role-of-mitochondria-in-me-cfs-13-july-2019/
The overview can be downloaded at the bottom of the page.
When I am well informed we have this finding even replicated. Fluge and Mella in 2016 (p. 15 in the 2019 ME Association mitochondria overview) and Prusty in 2020.
Rob Wüst is doing excellent work I think. What I found jaw dropping was that he came up with the simple but great idea to put a “deconditiong” control group to his study. I also remember that he is actively involved in the discussion with researchers who go on to claim that a psychiatric issue is at the core of ME/CFS.
But I doubt that Wüst will be able to find new valid and interesting hypotheses about the pathomechanism of ME/CFS. Which is simply due to the fact that the problems with the mitochondria are not the starting point of the ME/CFS inflammation processes.
I think that the immune system has been established as this starting point. I want to know is what ME/CFS trigger viral illnesses like COVID-19, the flu, glandular fever, ect. do to the immune system. Secondly, why it is that a T-cell pathology develops in ME/CFS.
So far we have two hypotheses what could go on. Pathological auto-immune processes and HHV-6b reactivation.
What I want to know is whether Rob Wüst’s findings about the pathological developments in the muscles could be explained by these viral or autoimmune inflammation processes.
Bonjour Cort. J’ai été diagnostiqué avec le FCS par le Dr. Byron Hyde Dr., d’Ottawa, Canada.
Malheureusement ce dernier est décédé il y a quelques mois! Celui-ci était un spécialiste et une sommité dans le domaine de la M.E/CFS. . autant au Canada qu’à travers le monde!
Suite à ce reportage j’aimerais vous informer que les tests déterminants pour poser le DX furent entre autres; Volume sanguin total qui démontrait une diminution des volumes plasmatiques érythrocytaires et sanguin inférieure à la normale soit plus ou moins 20%!
Également plusieurs Spects cérébraux anormaux démontrant un déficit au niveau des régions frontales bilatérales. Vous pourrez constater que les secteurs affectés occasionnent plusieurs symptômes similaires à ce que l’on retrouvent dans la maladie DFT!
Espérant ces infos pertinentes pour faire avancer la cause.
After many years of speculation, I still believe we need more research into non cytolytic viral infections that triggers mitochondrial dysfunction. Whether it is attributable to enteroviruses or reactivation of latent herpes viruses. Now that we have additional tools such as CRISPR/Cas13. It could help with diagnosis as well as treatment. Has Dr Chia retired from MECFS field? Perhaps Maureen Hansen could pursue additional research?
A team in London led by Jacqueline Cliff at Brunel researches the HHV-6b hypothesis.
https://www.brunel.ac.uk/research/projects/reactivation-of-herpesviruses-in-chronic-fatigue-syndrome